Few will deny that there is a need for better therapeutic alternatives to treat patients with hepatocellular carcinoma (HCC). Liver transplantation is among the best options for achieving long-term survival, with 60% to 70% of patients remaining alive at 5 years post-transplant.1,2 Unfortunately, patients with advanced HCC will not meet liver transplant criteria and, for HCC patients who do, wait times can be lengthy — often reaching more than 2, 3 or even 5 years.3 Prolonged wait time beyond 6 to 12 months can be a risk factor for tumor spread and dropout from the waiting list before transplantation.4
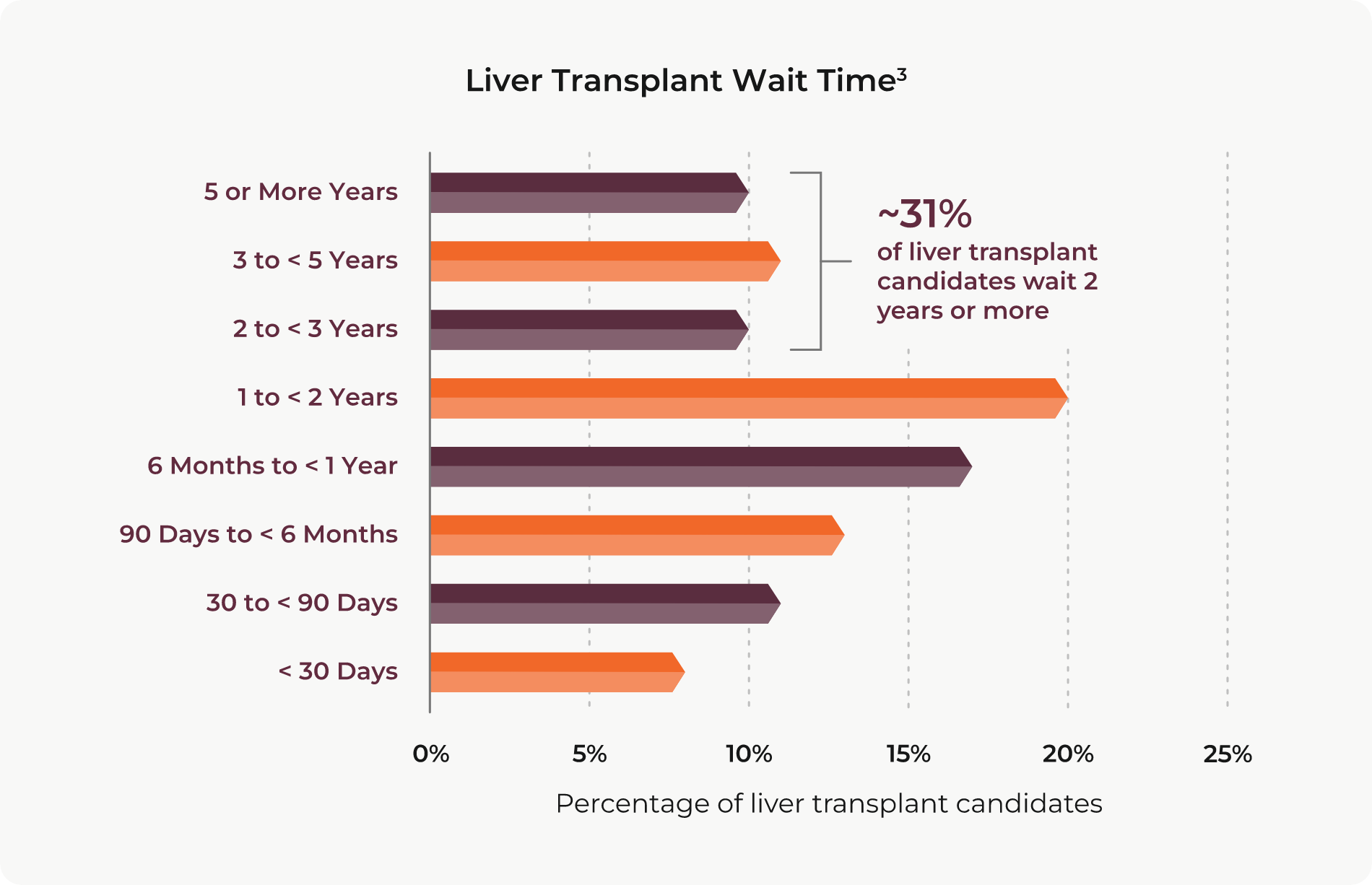
Successful locoregional therapy may make it possible to downstage ineligible patients so they can meet transplant criteria and to bridge eligible patients through the waiting period.5 Additionally, data shows that achieving complete response to pre-transplant locoregional therapy has the potential to improve post-transplant outcomes for patients with HCC, including reduced recurrence rate and a survival advantage.6,7
Help enable liver tumor downstaging and bridging to transplant.
Whether the goal is tumor downstaging or bridging to transplant, using the TriNav Infusion System with SmartValve® technology to deliver locoregional therapy via the Pressure-Enabled Drug Delivery™ (PEDD™) approach may help support better outcomes in this patient population.
The findings in two retrospective studies of patients with HCC who underwent transarterial chemoembolization (TACE) using the PEDD approach support its potential role in helping patients attain their transplant goal.8,9
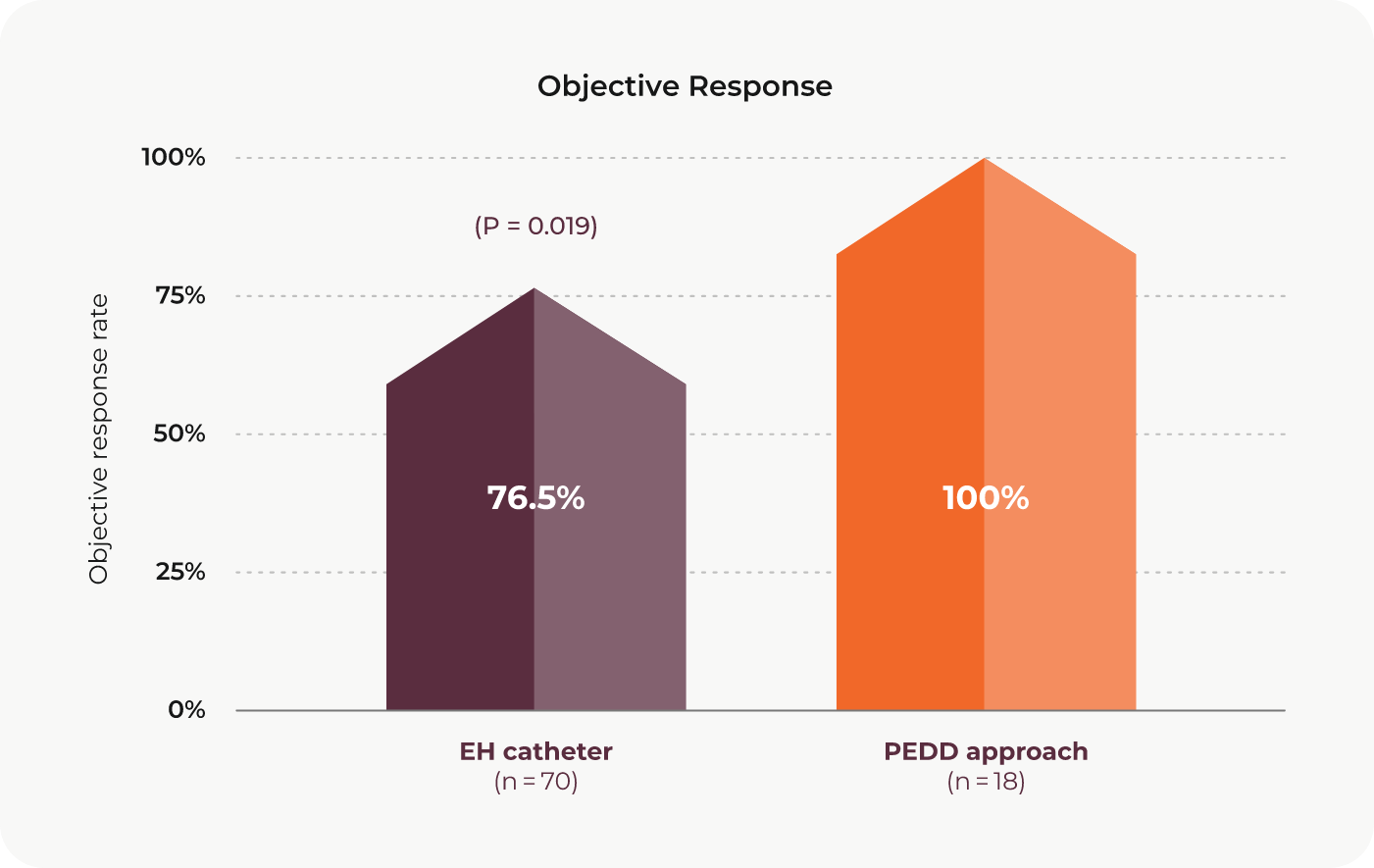
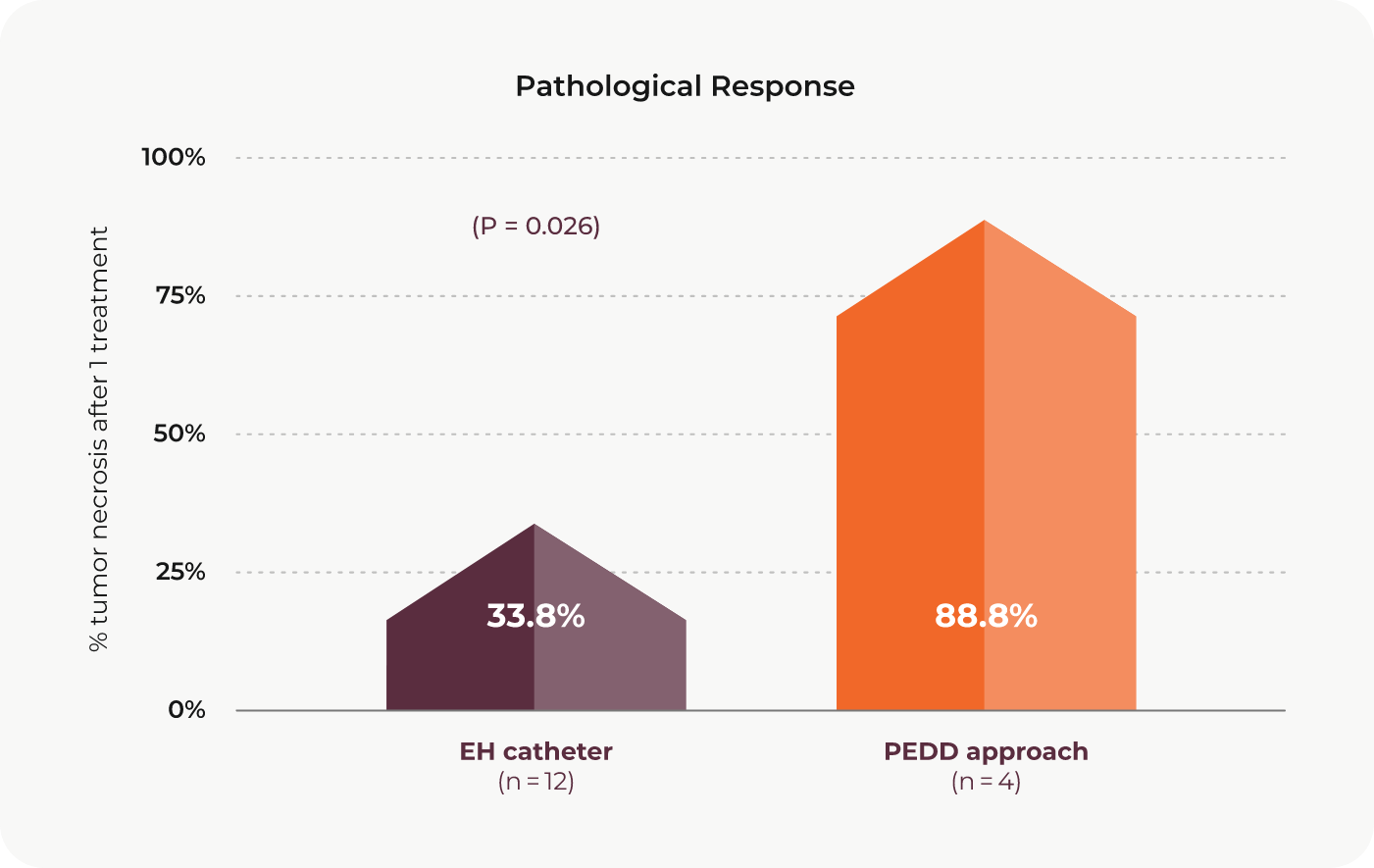
Study 1 (88 patients)8: Objective and pathological responses comparing the PEDD approach to an end-hole (EH) catheter
This study by Dr. Joseph J. Titano and colleagues, published in CardioVascular and Interventional Radiology, compared HCC tumor response to DEM-TACE treatment using the PEDD approach to response to DEM-TACE treatment using a traditional end-hole (EH) catheter. The objective response rate was significantly greater in the PEDD group relative to the EH group. For patients in the study who received a liver transplant, the pathological response, defined as percentage of tumor necrosis after one treatment, was also significantly greater in the PEDD group.
Although there was no statistically significant difference between the groups, nearly two-thirds of the PEDD group achieved complete response at one month.
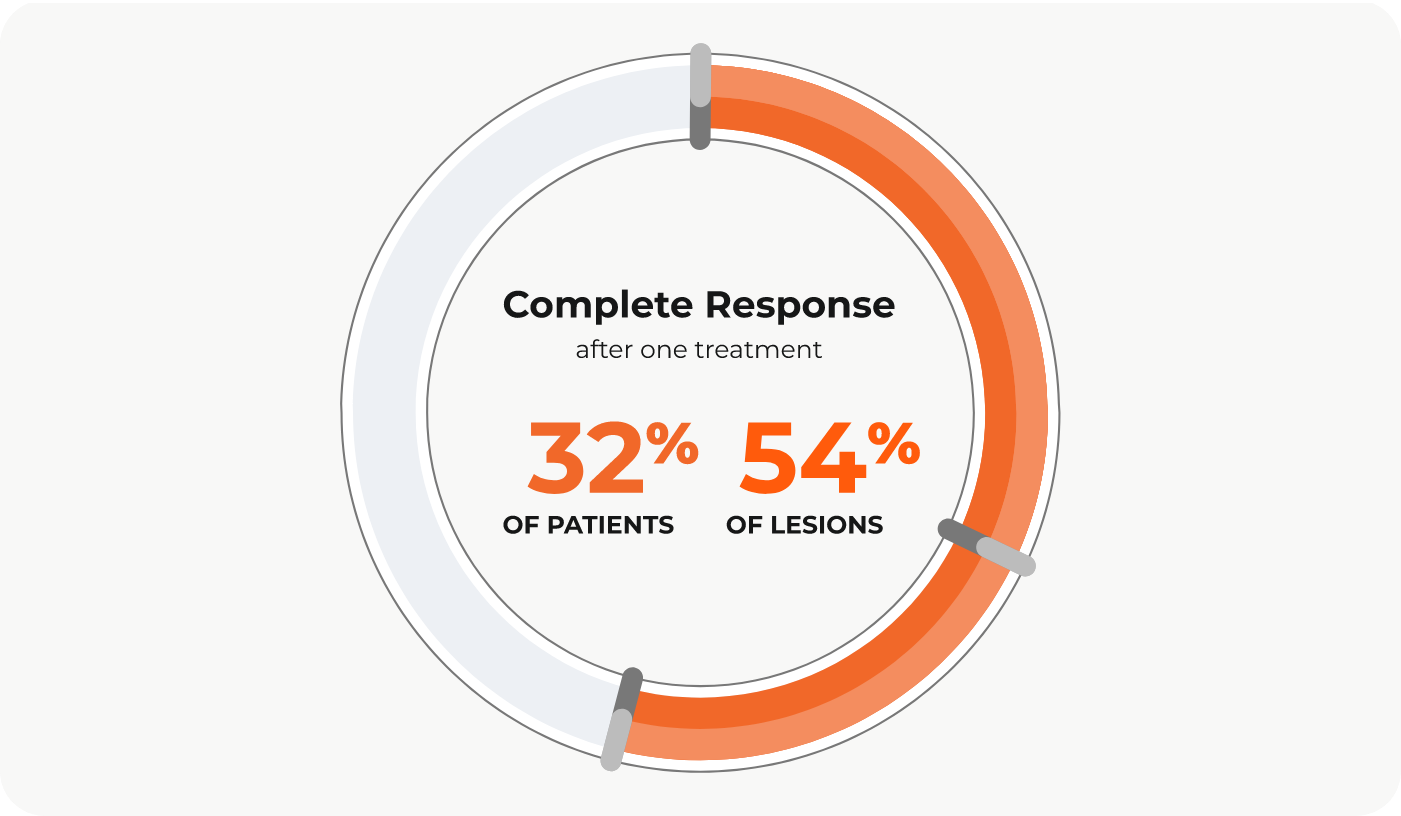
Study 2 (22 patients with 39 lesions)9: Complete response and downstaging results with the PEDD approach
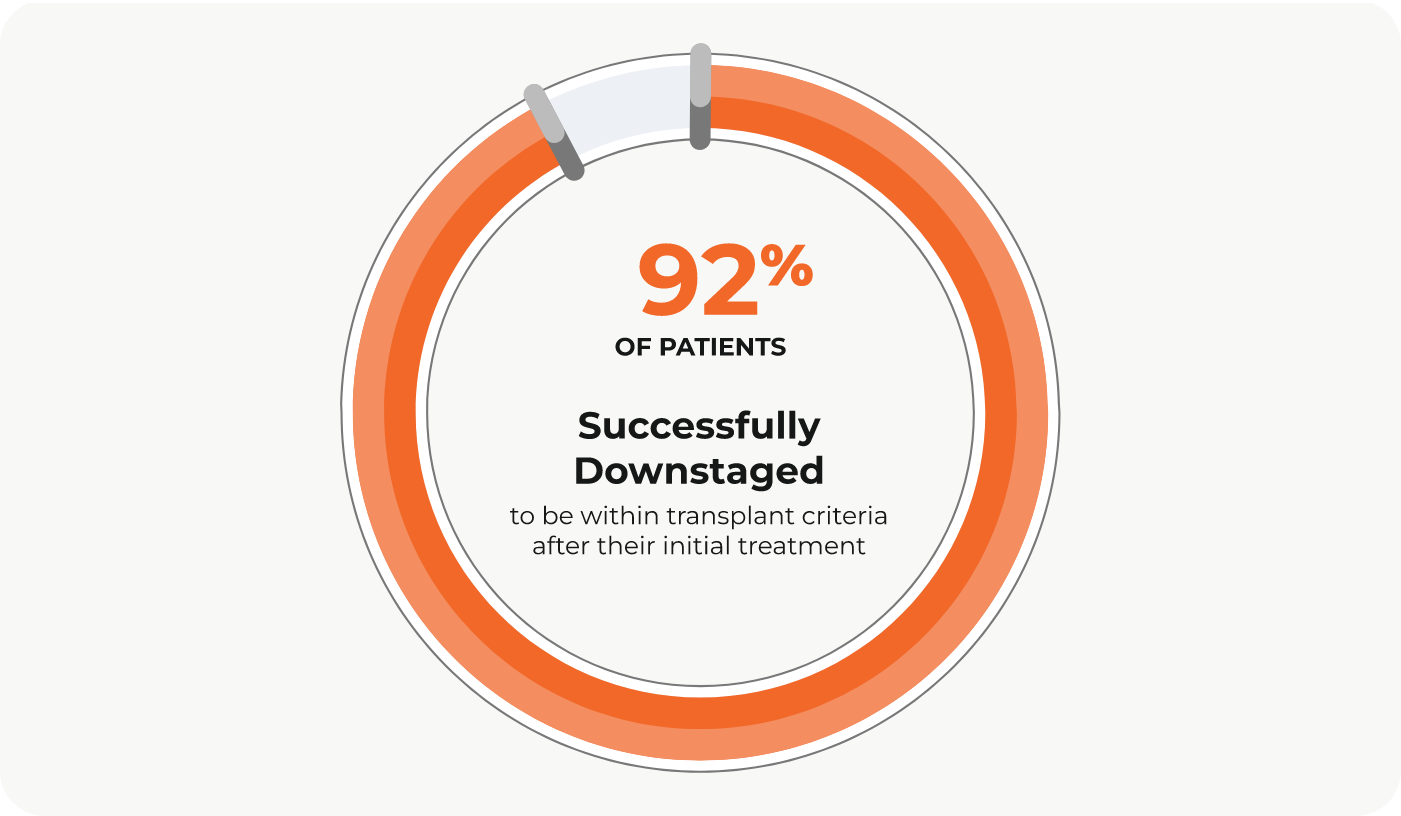
Dr. Alexander Y. Kim and colleagues published a retrospective study in PLoS One that looked at tumor response in patients with HCC who underwent DEE-TACE using the PEDD approach. After just one treatment, complete response was achieved in 32% of patients and 54% of lesions. Additionally, of the patients who were ineligible for transplant at the time of treatment, 21 of 22 patients (92%) had their disease successfully downstaged to be within transplant criteria after their initial treatment.
Bridge therapy or tumor downstaging using the PEDD approach may offer liver tumor patients new hope for transplant.
Published data on locoregional TACE using the PEDD approach supports the potential role of the TriNav Infusion System in helping patients with HCC reach their transplant goal, and the opportunity to achieve complete response may position them for improved post-transplant outcomes and a survival advantage.
Rx Only. For the safe and proper use of the TriNav device, refer to the Instructions for Use.
Indications for Use: The TriNav Infusion System is intended for use in angiographic procedures. It delivers radiopaque media and therapeutic agents to selected sites in the peripheral vascular system.10
Contraindications: TriNav is not intended for use in the vasculature of the central nervous system
(including the neurovasculature) or central circulatory system (including the coronary vasculature).10
REFERENCES:
1. Galuppo R, McCall A, Gedaly R. The role of bridging therapy in hepatocellular carcinoma. Int J
Hepatol. 2013;2013:419302.
2. Xing M, Kim HS. Independent prognostic factors for post-transplant survival in hepatocellular
carcinoma patients undergoing liver transplantation. Cancer Med. 2017;6(1):26-35.
3. US Department of Health and Human Services. Organ Procurement and Transplantation Network.
https://optn.transplant.hrsa.gov/data/view-data-reports/build-advanced/#. Accessed October 16,
2022.
4. Pompili M, Francica G, Ponziani FR, Iezzi R, Avolio AW. Bridging and downstaging treatments for hepatocellular carcinoma in patients on the waiting list for liver transplantation. World J Gastroenterol. 2013;19(43):7515-7530.
5. Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450-1462.
6. DiNorcia J, Florman SS, Haydel B, et al. Pathologic response to pretransplant locoregional therapy is
predictive of patient outcome after liver transplantation for hepatocellular carcinoma: analysis from the
US Multicenter HCC Transplant Consortium. Ann Surg. 2020;271(4):616-624.
7. Kim BK, Kim SU, Kim KA, et al. Complete response at first chemoembolization is still the most robust
predictor for favorable outcome in hepatocellular carcinoma. J Hepatol. 2015;62(6):1304-1310.
8. Titano JJ, Fischman AM, Cherian A, et al. End-hole versus microvalve infusion catheters in patients
undergoing drug-eluting microspheres-TACE for solitary hepatocellular carcinoma tumors: a
retrospective analysis. Cardiovasc Intervent Radiol. 2019;42(4):560-568.
9. Kim AY, Frantz S, Krishnan P, et al. Short-term imaging response after drug-eluting embolic transarterial chemoembolization delivered with the Surefire Infusion System® for the treatment of
hepatocellular carcinoma. PLoS One. 2017;12(9):e0183861.
10. TriSalus TriNav Infusion System, Instructions for Use.