 Here’s something you and your interventional radiologist colleagues may never have learned in medical school: The most potent state-of-the-art therapeutic option for defeating a solid tumor may not achieve the hoped-for result unless it is delivered by an equally advanced method of drug delivery — because otherwise it may not actually reach deep into the tumor. This is particularly true of liver tumors.
Here’s something you and your interventional radiologist colleagues may never have learned in medical school: The most potent state-of-the-art therapeutic option for defeating a solid tumor may not achieve the hoped-for result unless it is delivered by an equally advanced method of drug delivery — because otherwise it may not actually reach deep into the tumor. This is particularly true of liver tumors.
Why is that? One key reason is intratumoral pressure.
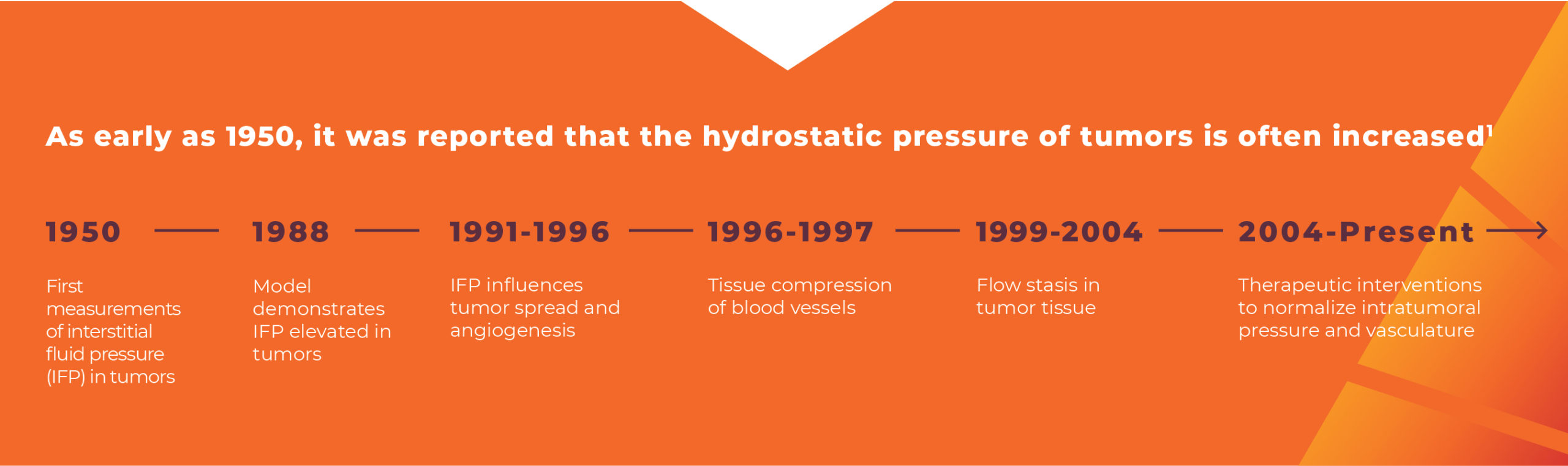
Intratumoral pressure is not a well-recognized barrier to therapy delivery, even though the concept is nothing new. Since the 1950s, researchers have been publishing about how the hydrostatic pressure of tumors is often increased.1 But it’s true that intratumoral pressure is difficult to measure, occurs in vivo, and therefore is often overlooked in molecular-biology-driven cancer research.2 Several therapeutic approaches have been tried to lower intratumoral pressure, but none have had a lasting impact in clinical practice.2
How Intratumoral Pressure Affects Therapy Delivery
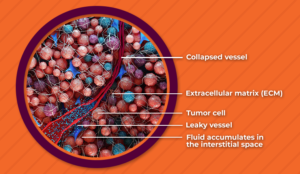 High intratumoral pressure is a function of two physiologic forces in the tumor microenvironment.
High intratumoral pressure is a function of two physiologic forces in the tumor microenvironment.
Elevated interstitial fluid pressure can limit the ability of drugs to leave blood vessels and deeply penetrate the tumor tissue.3 Leaky blood vessels cause fluid to seep into the interstitial space with no way out, and the lymphatic system within tumors is often underdeveloped and cannot drain fluids away. This leads to heterogeneous drug distribution within the tumor.4
Solid stress collapses blood vessels, often preventing anticancer drugs from reaching malignant cells downstream.5 The proliferation of the fibrous extracellular matrix and cells in the tumor results in the development of solid stress, and solid stress compresses vessels, which may reduce or halt blood flow to many parts of the tumor.6
Physiologic pressure from the cardiac cycle is insufficient to overcome intratumoral pressure.7 So, then, how can interventional radiologists outsmart it?
How You Can Outsmart Intratumoral Pressure
Our solution to outsmarting intratumoral pressure is this: Consider using the TriNav® Infusion System with SmartValve® technology to deliver therapy. The TriNav Infusion System is powered by the Pressure-Enabled Drug Delivery™ (PEDD™) approach, which increases intravascular pressure beyond what the cardiovascular system can generate on its own,8 in order to help open collapsed vessels in the tumor and enable deeper perfusion.9,10
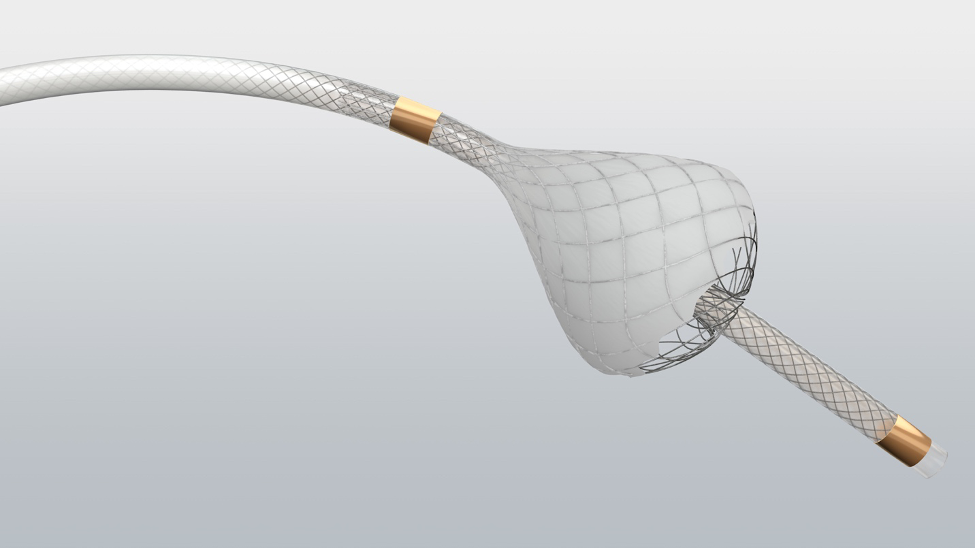
Your patients with liver cancer deserve the best treatment options. For patients with hepatocellular carcinoma and liver metastases, evidence supports the use of the TriNav Infusion System and its PEDD approach to therapy administration to improve their opportunity for potentially better outcomes.9,11,12
Outsmart intratumoral pressure for your patients with the TriNav Infusion System with SmartValve technology. It’s the smart thing to do.
REFERENCES
- Young JS, Lumsden CE, Stalker AL. The significance of the tissue pressure of normal testicular and of neoplastic (Brown-Pearce carcinoma) tissue in the rabbit. J Pathol Bacteriol. 1950;62(3):313-333.
- Böckelmann LC, Schumacher U. Targeting tumor interstitial fluid pressure: will it yield novel successful therapies for solid tumors? Expert Opin Ther Targets. 2019;23(12):1005-1014.
- Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure – an obstacle in cancer therapy. Nat Rev Cancer. 2004;4(10):806-813.
- Sheth RA, Hesketh R, Kong DS, Wicky S, Oklu R. Barriers to drug delivery in interventional oncology. J Vasc Interv Radiol. 2013;24(8):1201-1207.
- Stylianopoulos T, Martin JD, Chauhan VP, et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc Natl Acad Sci U S A. 2012;109(38):15101-15108.
- Jain RK. An indirect way to tame cancer. Sci Am. 2014;310(2):46-53.
- Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol. 2013;31(17):2205-2218.
- Data on file (CEA 001 trial). TriSalus Life Sciences, 2019.
- Titano JJ, Fischman AM, Cherian A, et al. End-hole versus microvalve infusion catheters in patients undergoing drug-eluting microspheres-TACE for solitary hepatocellular carcinoma tumors: a retrospective analysis. Cardiovasc Intervent Radiol. 2019;42(4):560-568.
- Data on File. REP-0362. TriSalus Life Sciences, 2021.
- Pasciak AS, McElmurray JH, Bourgeois AC, Heidel RE, Bradley YC. The impact of an antireflux catheter on target volume particulate distribution in liver-directed embolotherapy: a pilot study. J Vasc Interv Radiol. 2015;26(5):660-669.
- Katz SC, Moody AE, Guha P, et al. HITM-SURE: Hepatic immunotherapy for metastases phase Ib anti-CEA CAR-T study utilizing pressure enabled drug delivery. J Immunother Cancer. 2020;8(2):e001097.