A Revolution in Precision Embolization
The TriNav® Infusion System with SmartValve® technology leverages the Pressure-Enabled Drug Delivery™ (PEDD™) approach which can help overcome challenges that impede therapeutic delivery to the tumor and improve the T:N ratio.1-3
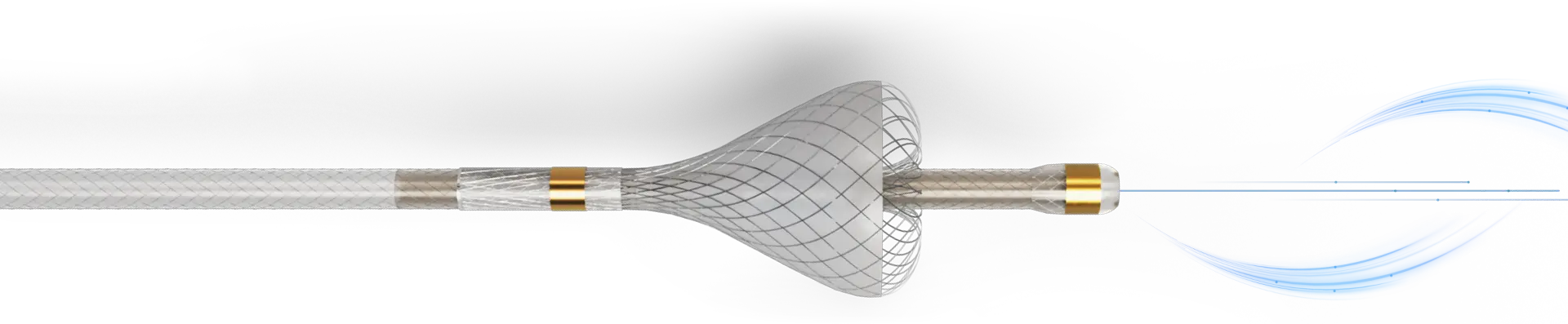
Traditional delivery methods may fall short
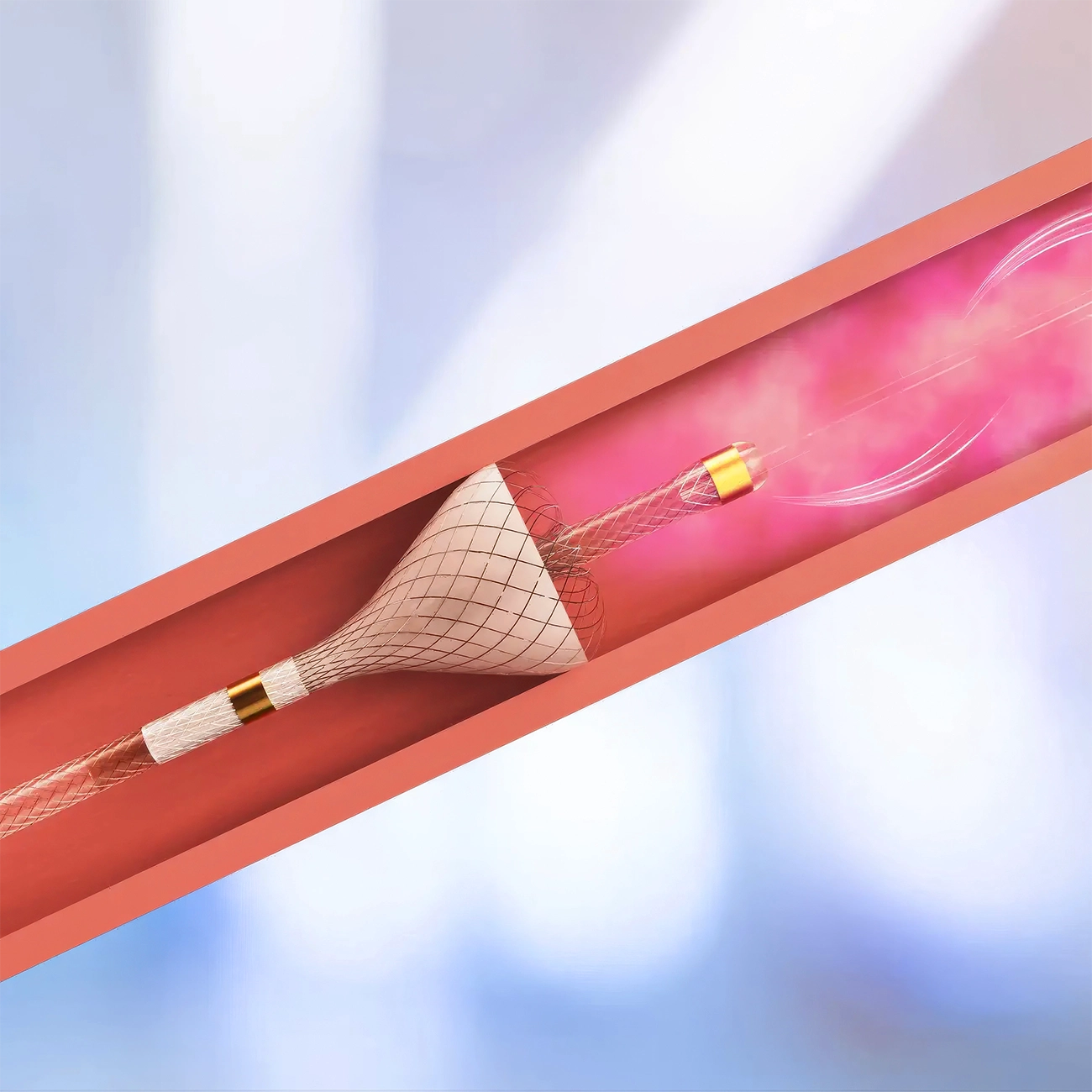
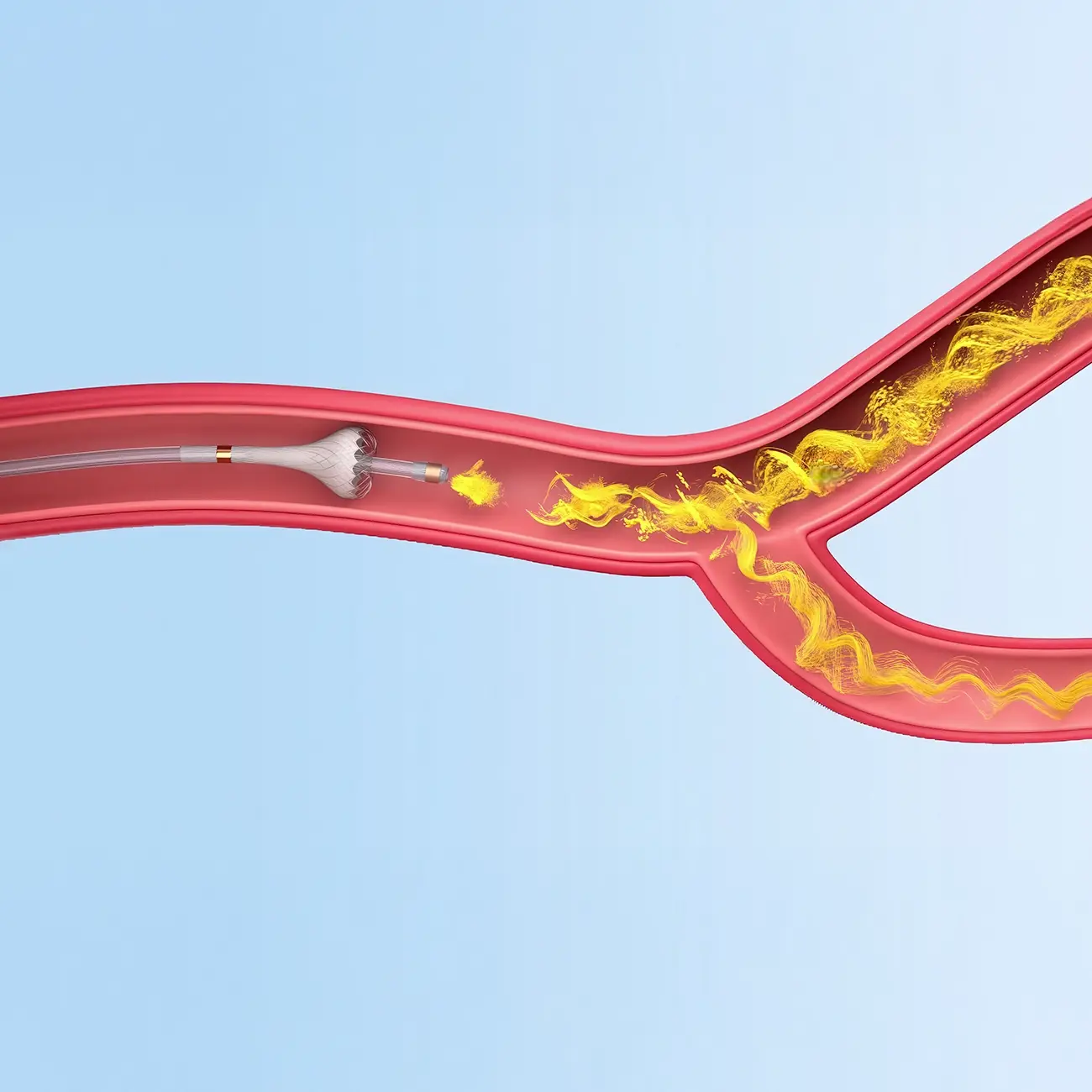
Traditional microcatheters can have difficulty overcoming variables that can impede drug delivery, like flow dynamics and intratumoral pressure.4 With SmartValve technology, TriNav Infusion Systems can overcome barriers that can inhibit successful treatment.1-3
Traditional Microcatheter
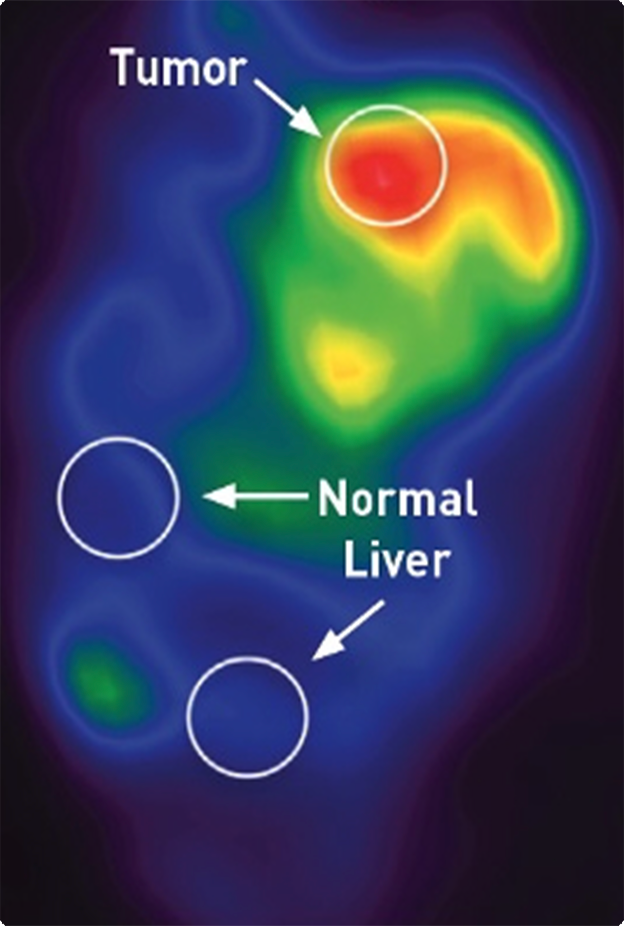
Therapy distribution with a traditional microcatheter.2
SmartValve Technology
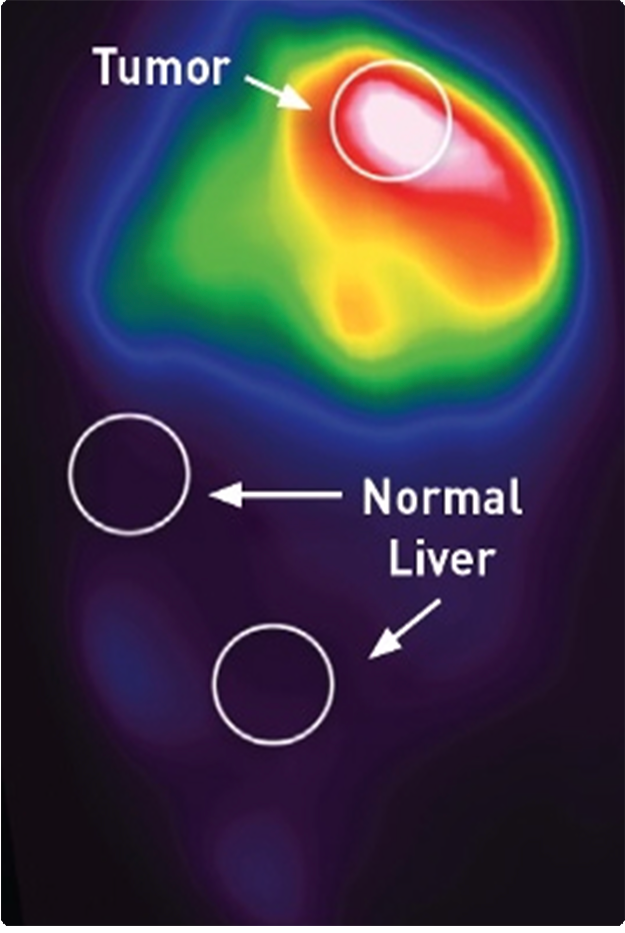
Therapy distribution with SmartValve technology.2
Why use it
Here’s how TriNav is changing the game in therapy delivery:
Engineered for Precision
24% increase in the T:N ratio compared to traditional microcatheters (n=61, p<0.001)1
Designed for Safety
Up to 58% mean decrease in non-target embolization and demonstrated reflux protection compared to traditional microcatheters (n=9, p<0.05)2
Focused on Outcomes
Compared to patients treated with traditional microcatheters, clinical data show patients treated with SmartValve technology experienced:
- 23% increase in tumor dose (n=61, p=0.001)1
- 55% increase in pathological response rate (n=23, p=0.026)3
- 23% increase in objective response rate (n=88, p=0.019)3
- 61% lower rate of post-procedure in-patient visits (8.0% vs. 20.5%)5,6
- 5.7% improvement in complication rates5,6
Advancing Reimbursement and Access
The only device with unique CMS-assigned HCPCS codes for both simulation angiogram (C8004) and treatment (C9797), supporting reimbursement for procedural planning and therapy delivery.
- $7.7K cost avoidance per case through reductions in in-patient visits and treatment-related complications shown through Real-World Evidence.5,6
How it Works
SmartValve® Technology has three unique features
A key component of TriNav Infusion Systems is SmartValve technology which offers meaningful clinical benefits over traditional delivery methods.