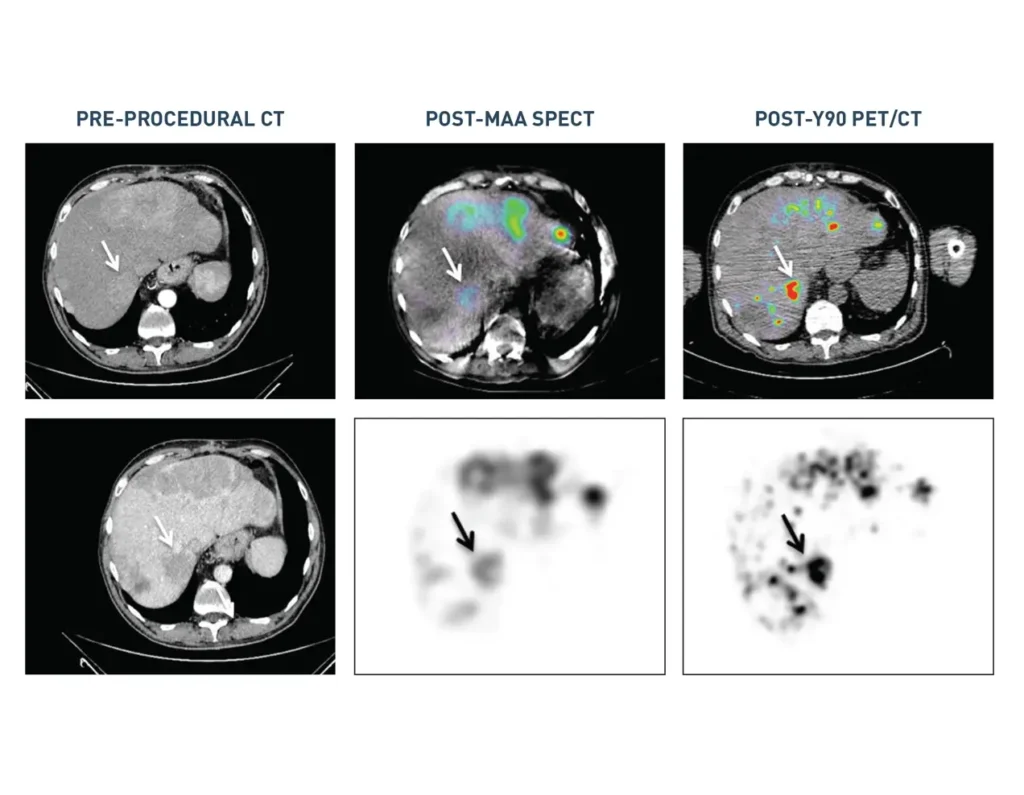
Clinical Data
Engineered Differently Than Traditional Microcatheters
SmartValve® Technology is clinically proven to more precisely target solid tumors, facilitate deeper therapy penetration, and protect against non-target embolization.1-3
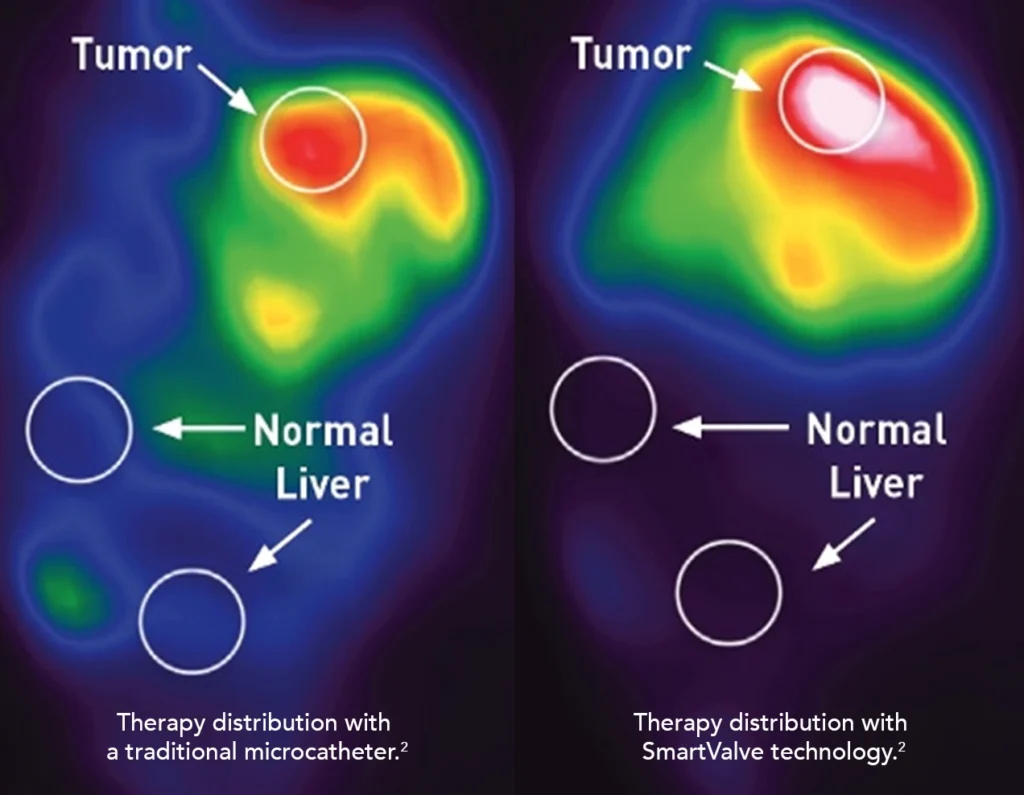
Gets more therapy precisely where you need it
Studies with liver cancer patients show that SmartValve® technology gets more therapy to the tumor than a traditional microcatheter.
- 34% increase in particles delivered to the tumor (n=23, p=0.002)3
- 23% increase in tumor dose (n=61, p<0.001)1
- 68% increase in tumor deposition (n=9, p<0.05)2
Delivers less to healthy tissue
TriNav helps protect healthy tissue from potentially harmful side effects of treatment compared to a traditional microcatheter.
- Up to 58% mean decrease in non-target embolization (n=9, p<0.05)2
- Demonstrates reflux protection2
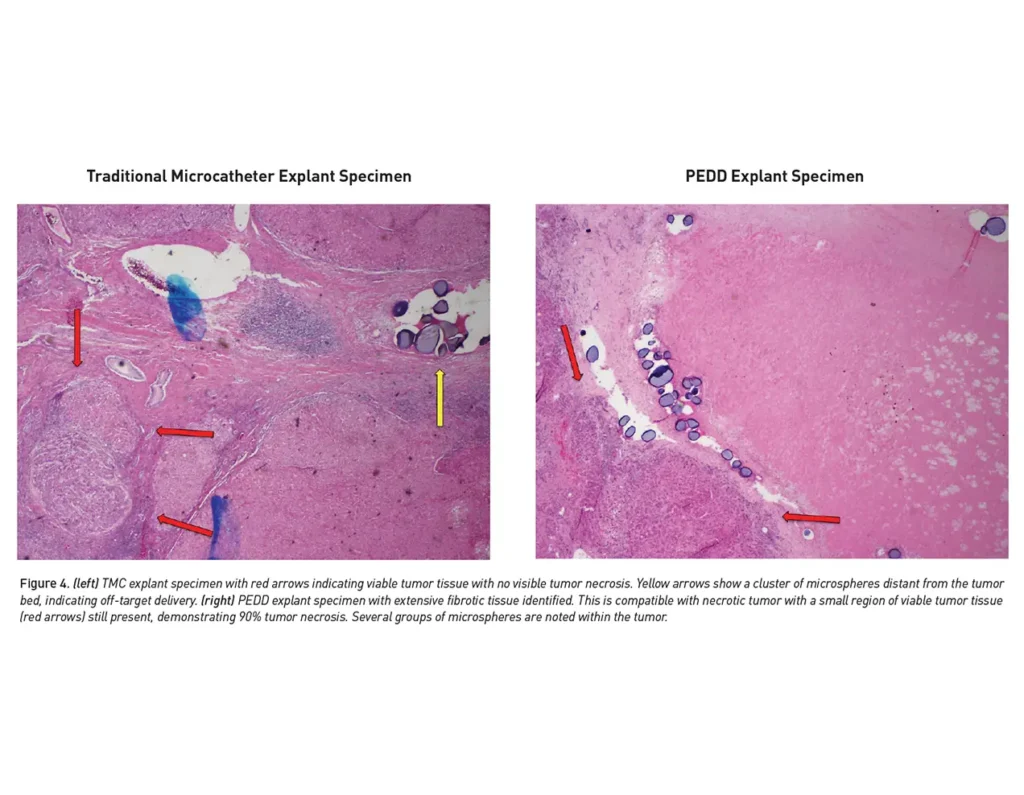
Better tumor response
More precise tumor targeting may result in a better response.
- 23% increase in overall response rate (100% vs. 77%; n=88; p=0.019)3
- 55% increase in pathological response rate (89% vs. 34%; n=23; p=0.026)3
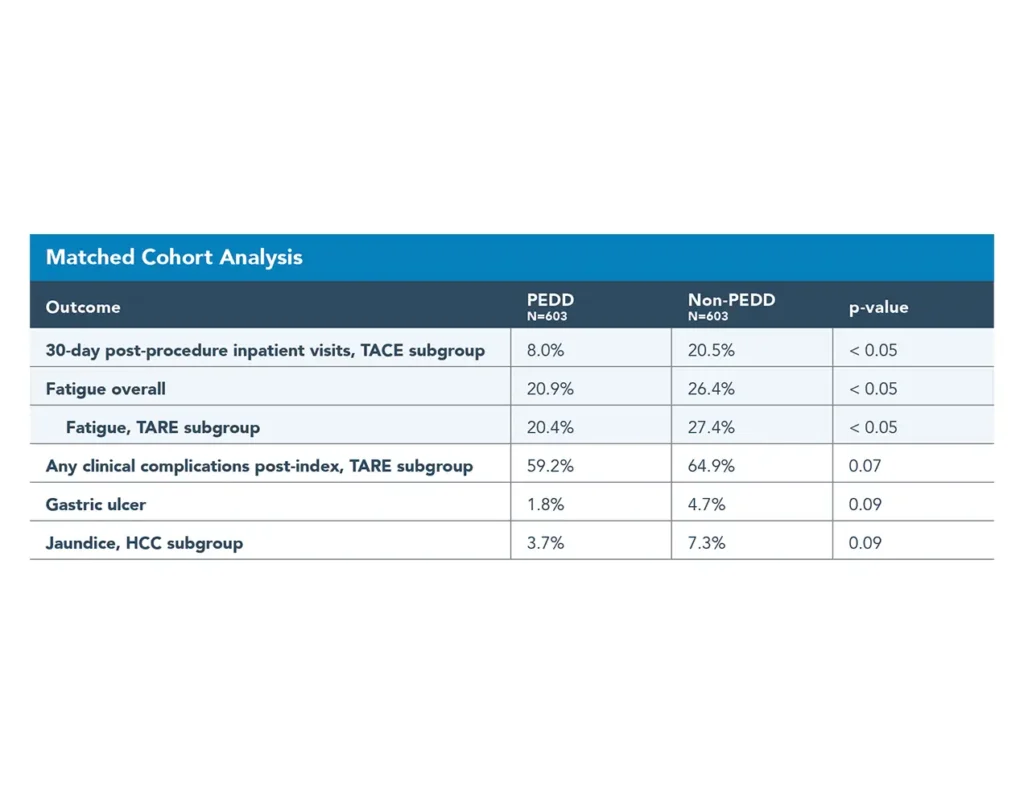
Improved clinical outcomes
Precision treatment can be more effective and may result in better clinical outcomes compared to a traditional microcatheter.
- 21% reduction in fatigue incidence (20.9% vs. 26.4%)4,5
- 49% reduction in reported jaundice (3.7% vs. 7.3%)4,5
- 61% reduction in gastric ulcer incidence (1.8% vs. 4.7%)4,5
- 61% lower rate of post-procedure in-patient visits (8.0% vs. 20.5%)4,5
- 5.7% improvement in complication rates4,5

Lower costs
In a comprehensive Real-World Evidence study, the benefits of SmartValve technology procedures translated to lower resource burden due to reduced hospitalizations and complications.
Learn more about our TriNav financial advantage.
- $3,135 reduced in-patient visits4,5
- $4,599 reduced charges related to clinical complications4,5
Indications For Use
The TriNav Infusion Systems are intended for use in angiographic procedures. They deliver radiopaque media and therapeutic agents to selected sites in the peripheral vascular system.
Contraindications
The TriNav Infusion Systems are not intended for use in the vasculature of the central nervous system (including the neurovasculature) or central circulatory system (including the coronary vasculature).
Rx Only
For the safe and proper use of the TriNav Infusion Systems, refer to their individual Instructions for Use.
References
- d’Abadie P, Walrand S, Goffette P, et al. Antireflux catheter improves tumor targeting in liver radioembolization with resin microspheres. Diagn Interv Radiol. 2021;27(6):768-773.
- Pasciak AS, McElmurray JH, Bourgeois AC, Heidel RE, Bradley YC. The impact of an antireflux catheter on target volume particulate distribution in liver-directed embolotherapy: a pilot study. J Vasc Interv Radiol. 2015;26(5):660-669.
- Titano JJ, Fischman AM, Cherian A, et al. End-hole versus microvalve infusion catheters in patients undergoing drug-eluting microspheres–TACE for solitary hepatocellular carcinoma tumors: a retrospective analysis. Cardiovasc Intervent Radiol. 2019;42(4):560-568.
- Cook K, Gupta D, Liu Y, et al. Real-world evidence of Pressure-Enabled Drug Delivery for trans-arterial chemoembolization and radioembolization among patients with hepatocellular carcinoma and liver metastases. Current Medical Research and Opinion. 2024;40(4):591-598.
- Gupta et al, Clinical Outcomes of Pressure-Enabled Drug Delivery for TACE and TARE; SIO- 2025 poster
*Tumor to normal ratio