How it Works
SmartValve® is Unique to TriNav®
The TriNav Infusion System®, with SmartValve technology, helps to precisely target the tumor and facilitates deeper therapy penetration while protecting against non-target embolization and reflux.1-3
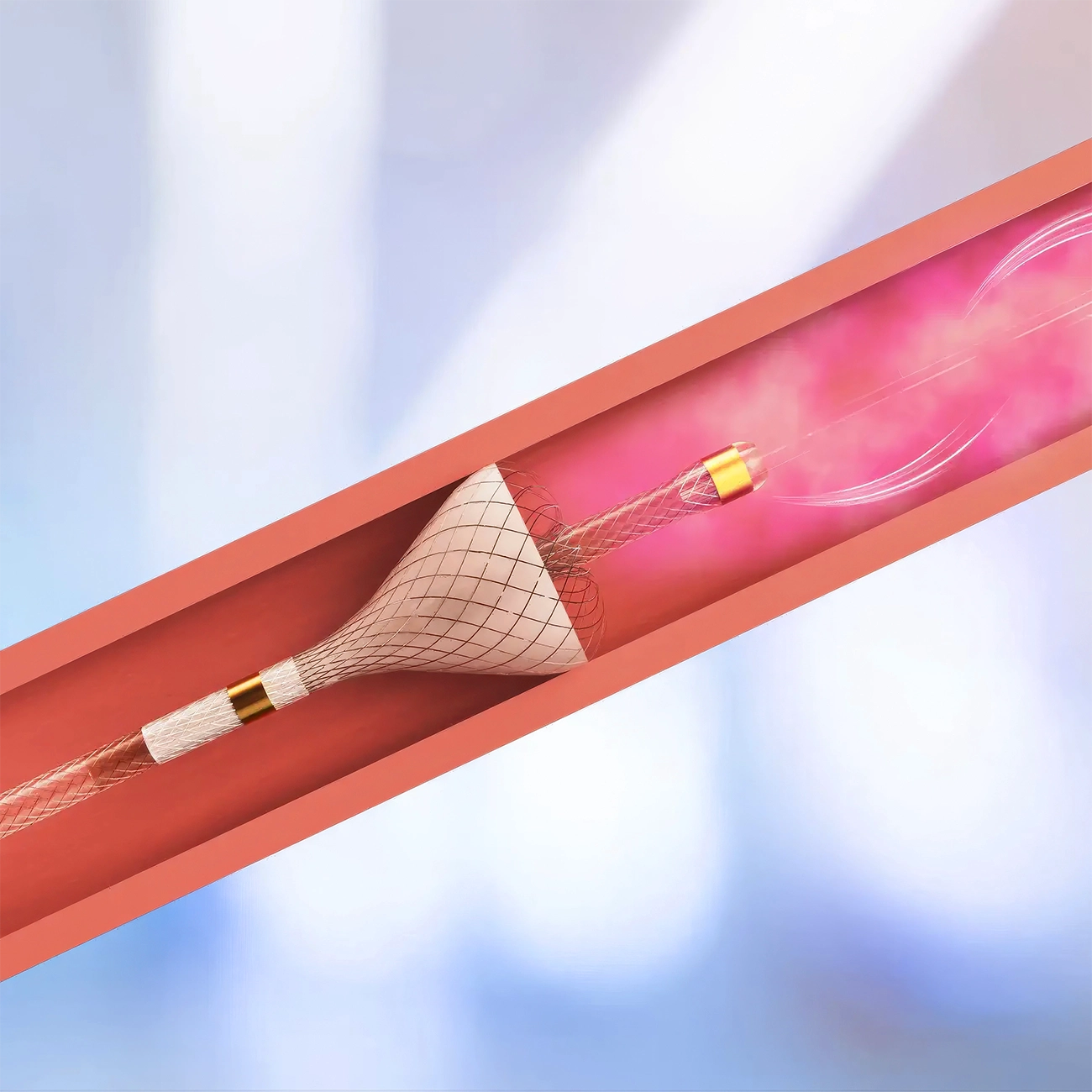
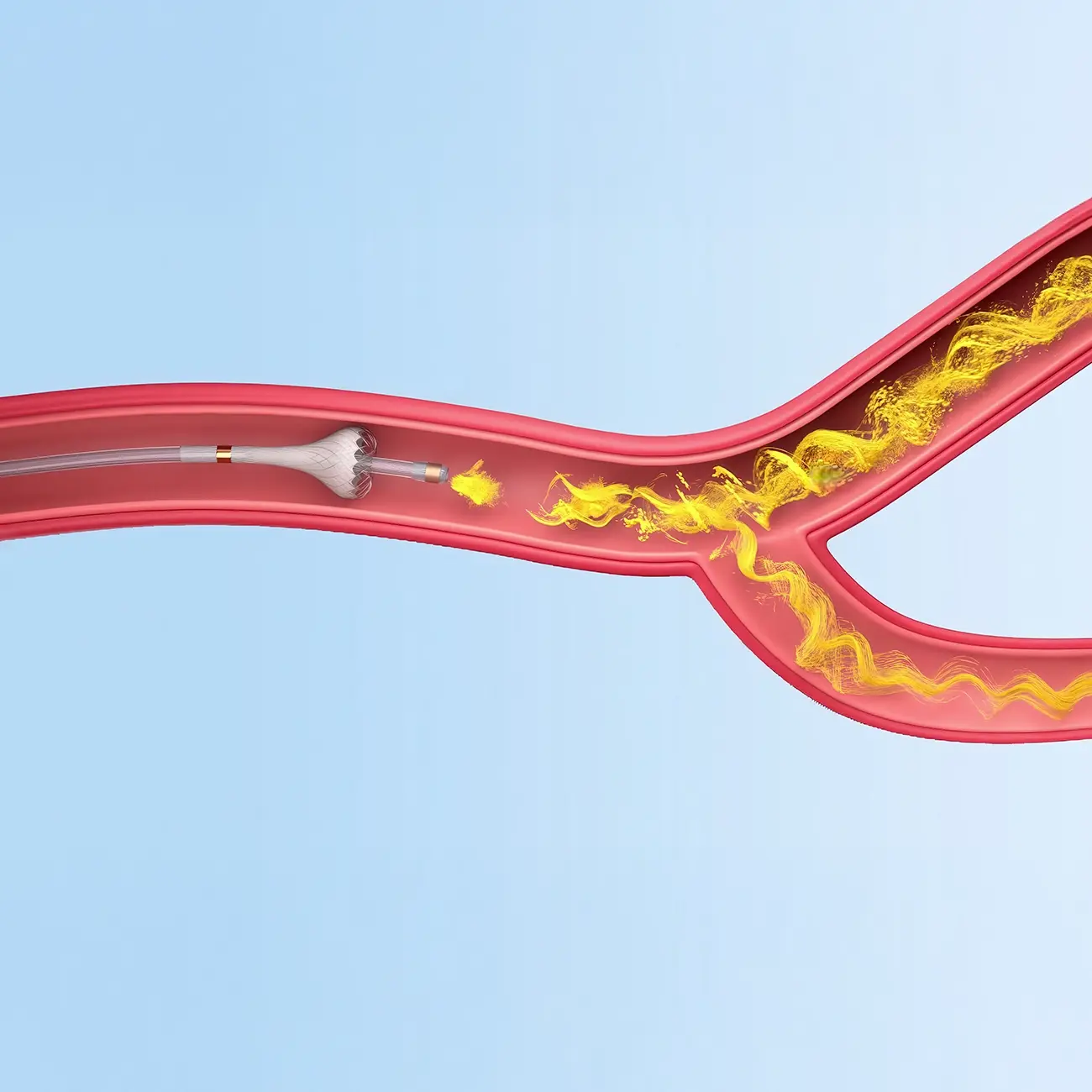
How SmartValve works
Three unique features translate into clear benefits
In quantitative bench-top testing, the SmartValve ensured fixed centro-luminal positioning in 100% of the experiments, vs. 29% when the traditional microcatheter was used.4
In quantitative bench-top testing, SmartValve Technology was shown to consistently induce a turbulent flow pattern which promotes mixing of microspheres into the bloodstream which led to a 62% improvement in downstream microsphere administration.4
SmartValve Technology helps open collapsed vessels to improve tumor penetration. Blinded histopathologic evaluation of explanted livers following DEB‑TACE to treat HCC showed that SmartValve technology achieved a 33% increase to beads in the tumor compared to a traditional microcatheter.3
Frequently asked questions
Learn more about TriNav Infusion Systems
TriNav has several options to choose from depending on your unique needs, with a portfolio of devices that give vessel size coverage from 1.5-5mm. Additionally, the TriNav FLX and TriNav XP feature a flexible distal tip to reach more challenging anatomy and the TriNav LV and TriNav XP are compatible with larger particles (beads ≤ 700 μm). These options help provide a unique tool specific to each case.
Recommended accessories include the appropriate sheath introducer and guide catheter, straight tip or curved guidewire, Hemostasis Valve, heparinized saline or equivalent flushing solution, and Luer Lock syringes. Refer to the product Instructions for Use for a complete list of recommended accessories.
Yes, there are numerous case studies and real-world use cases showing the benefits of TriNav. Explore our resources page to see how TriNav is supporting better outcomes for patients with liver tumors.
| TriNav is the only device with CMS designated HCPCS codes for both simulation angiogram (C8004) and treatment (C9797) procedures. Combined with a partnership with ZHealth, an industry leader in coding and reimbursement support, TriNav gives you the resources you need to navigate the reimbursement process. Visit our reimbursement and economic advantages page to learn more about how you can make the most of your investment in TriNav Infusion System. |
| Yes. Hydrating the TriNav helps the coating become more lubricious, a process that takes 10-15 seconds. Proper hydration is crucial to maintain the coating’s effectiveness to ensure intended performance. Maintaining proper hydration of the TriNav may improve trackability, may help reduce the likelihood of thrombus formation, and supports clinician satisfaction. Additionally, when not in use, keep the TriNav hydrated in a heparinized saline bath. Refer to the Technical User Guide for details on how to properly hydrate the TriNav. |
Trackability is influenced by many factors that contribute to overall system stability such as:
For tips on improving trackability, refer to the Technical User Guide. |
Indications For Use
The TriNav Infusion Systems are intended for use in angiographic procedures. They deliver radiopaque media and therapeutic agents to selected sites in the peripheral vascular system.
Contraindications
The TriNav Infusion Systems are not intended for use in the vasculature of the central nervous system (including the neurovasculature) or central circulatory system (including the coronary vasculature).
Rx Only
For the safe and proper use of the TriNav Infusion Systems, refer to their individual Instructions for Use.
References
- d’Abadie P, Walrand S, Goffette P, et al. Antireflux catheter improves tumor targeting in liver radioembolization with resin microspheres. Diagn Interv Radiol. 2021;27(6):768-773.
- Pasciak AS, McElmurray JH, Bourgeois AC, Heidel RE, Bradley YC. The impact of an antireflux catheter on target volume particulate distribution in liver-directed embolotherapy: a pilot study. J Vasc Interv Radiol. 2015;26(5):660-669.
- Titano JJ, Fischman AM, Cherian A, et al. End-hole versus microvalve infusion catheters in patients undergoing drug-eluting microspheres–TACE for solitary hepatocellular carcinoma tumors: a retrospective analysis. Cardiovasc Intervent Radiol. 2019;42(4):560-568.
- van den Hoven A F, Lam M, Jernigan S, van den Bosch M, Buckner G D. Innovation in catheter design for intra-arterial liver cancer treatments results in favorable particle-fluid dynamics. J. Exp. Clin. Cancer Res. 2015; 34:74.
- 2024 Internal Competitive Market Analysis of Available Diagnostic and Guiding Catheters.