TriNav’s PEDD approach significantly increases the T:N ratio,* which may result in better patient outcomes.1,2,4
PEDD Improved Tumor Targeting in Liver Radioembolization with Resin Microspheres.2
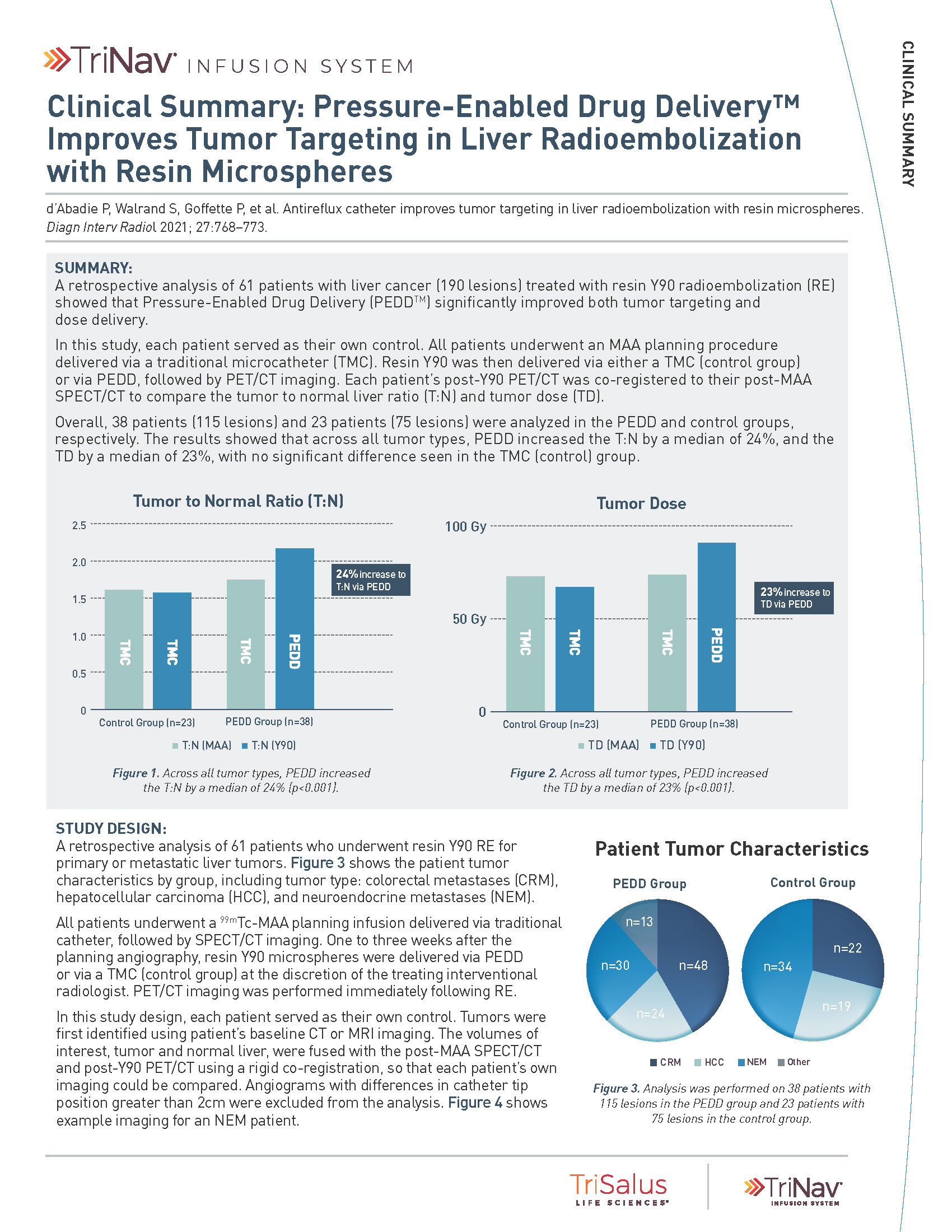
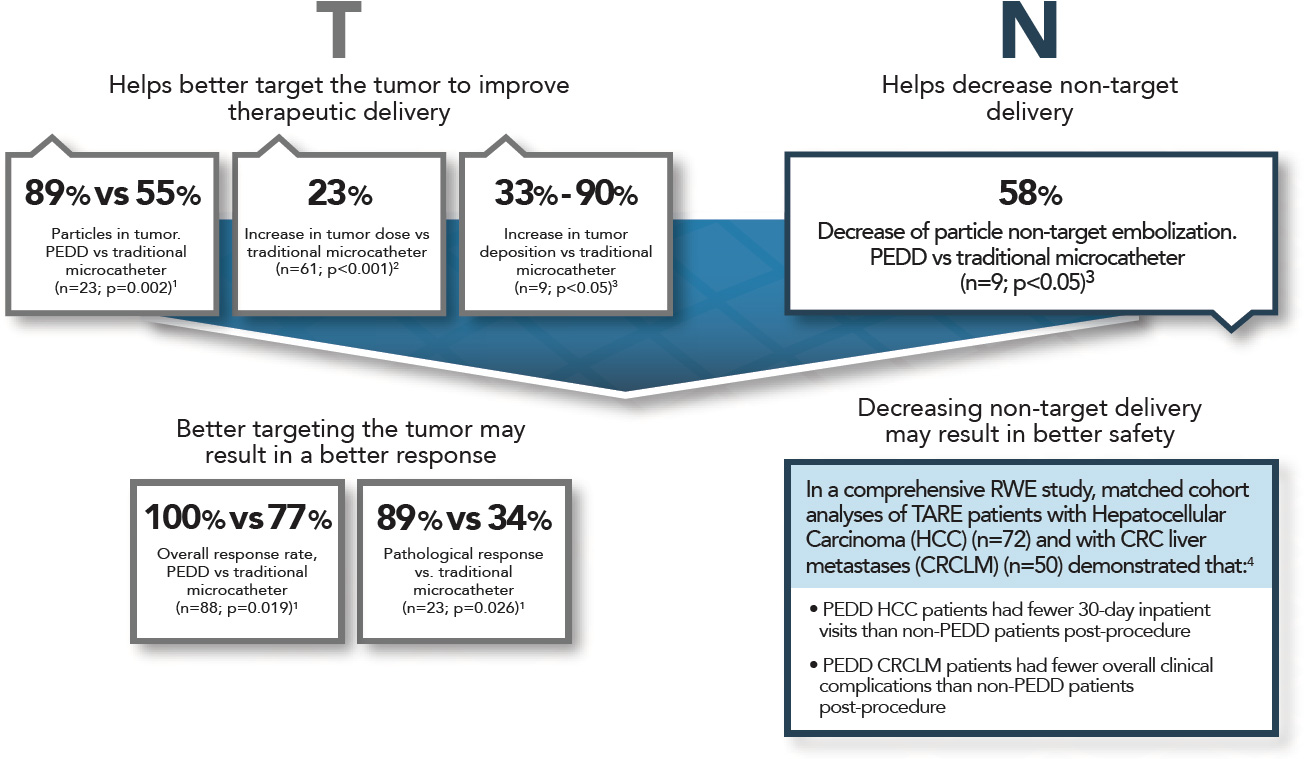
PEDD significantly increased both T:N Ratio and Tumor Dose compared to a traditional microcatheter (TMC).

24%
increase in T:N via PEDD
23%
increase in tumor dose via PEDD
Study Design
A retrospective analysis of 61 patients with liver cancer (190 lesions). All patients underwent an MAA planning procedure delivered via a traditional microcatheter. Resin Y90 was delivered via either an traditional microcatheter (control group) or via PEDD, followed by PET/CT imaging. Each patient’s post-Y90 PET/CT was co-registered to their post-MAA SPECT/CT to compare the T:N ratio and tumor dose.
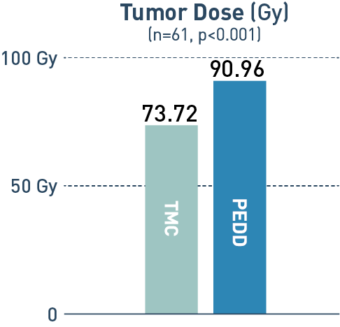
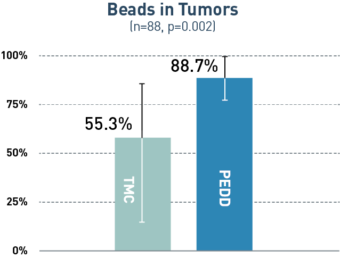
PEDD Significantly Increased Tumor Penetration and Response in DEM-TACE to Treat HCC.1
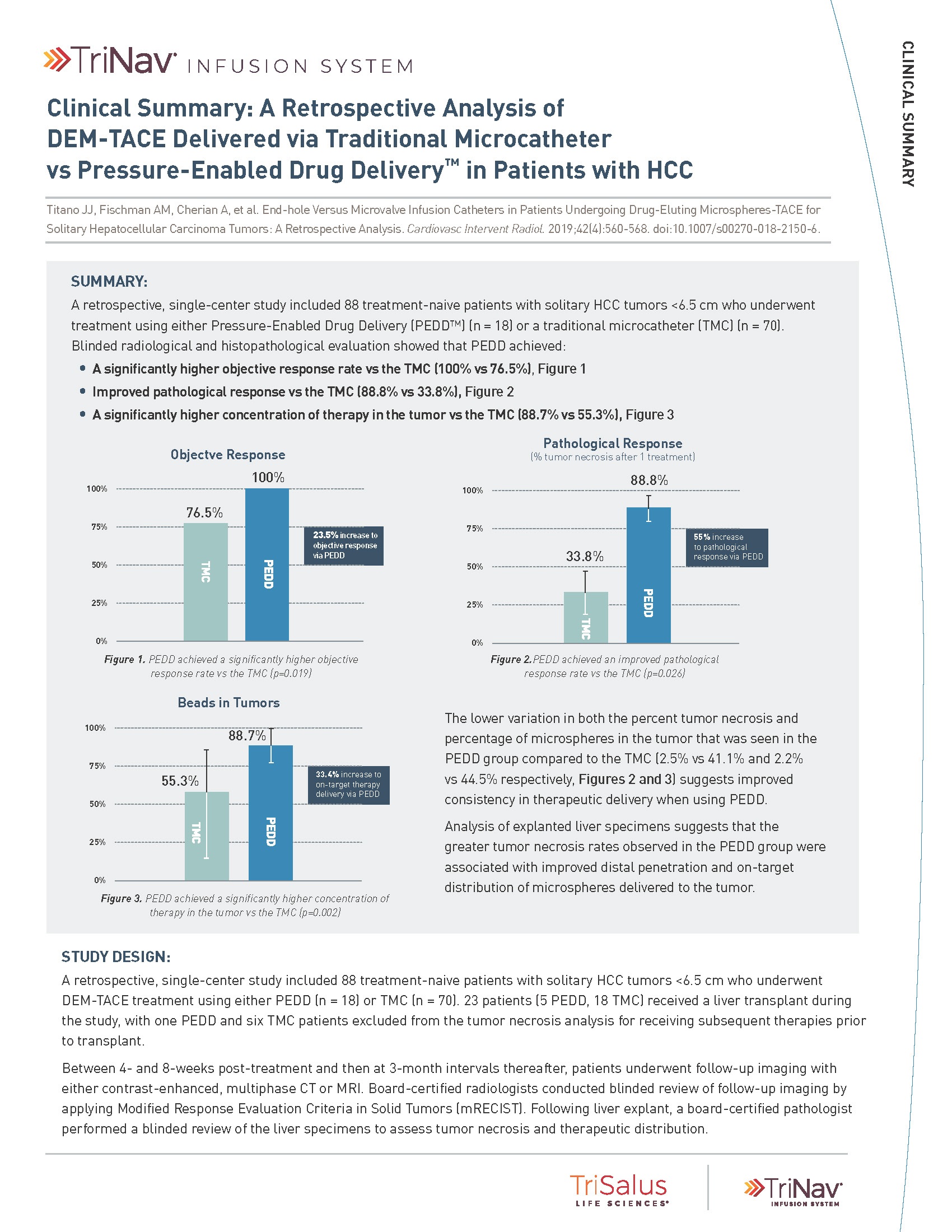
PEDD delivered a significantly higher concentration of therapy in the tumor compared to a traditional microcatheter (TMC).
33%
increase in on-target
therapy delivery
via PEDD
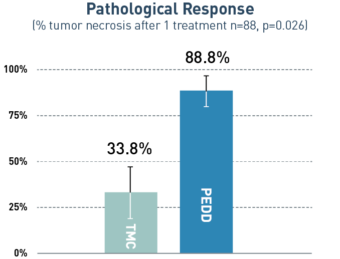
55%
increase in
pathological response
via PEDD
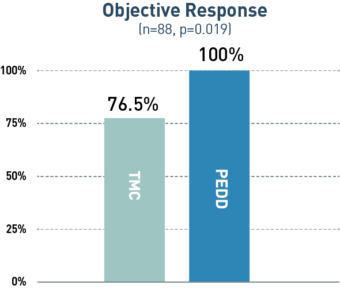
23%
increase in
objective response
via PEDD
Study Design
A retrospective, single-center study included 88 treatment-naive patients with solitary HCC tumors <6.5 cm who underwent treatment utilizing either PEDD (n=18) or a traditional microcatheter (n=70).
Board-certified radiologists conducted blinded review of follow-up imaging according to mRECIST. Following liver explant (n=23), a board-certified pathologist performed a blinded review of the liver specimens to assess tumor necrosis and therapeutic distribution.
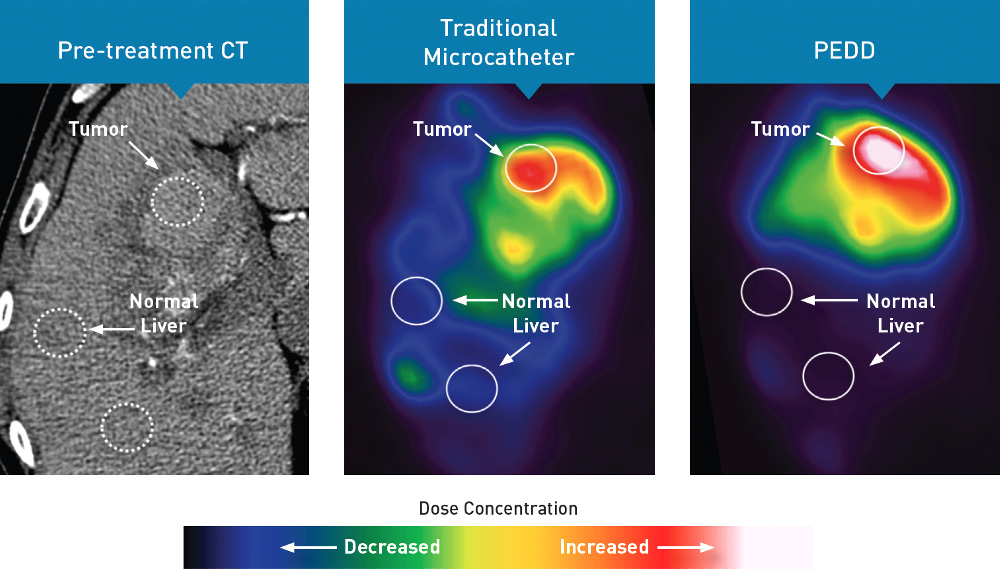
PEDD Significantly Improved Tumor Targeting Compared to a Traditional Microcatheter.3
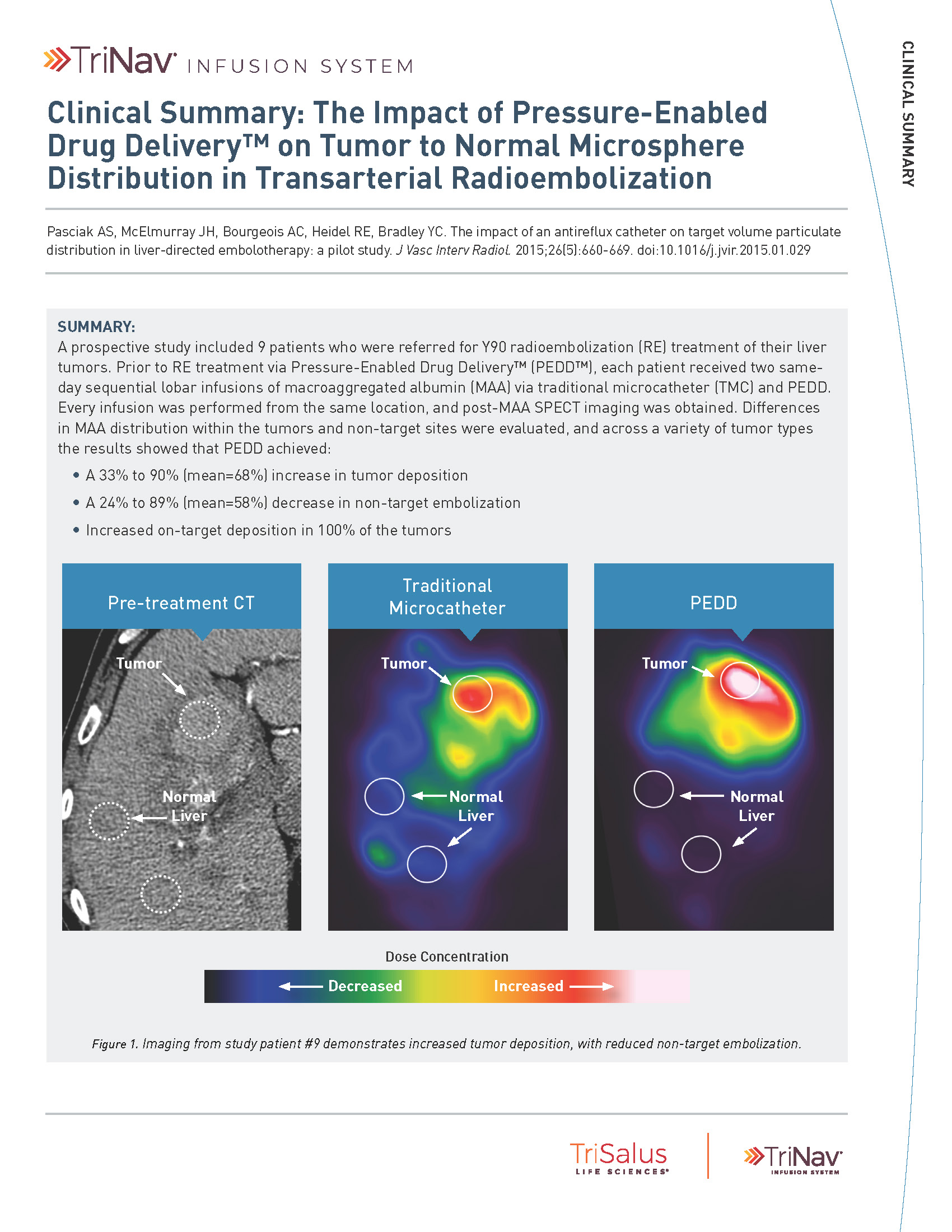
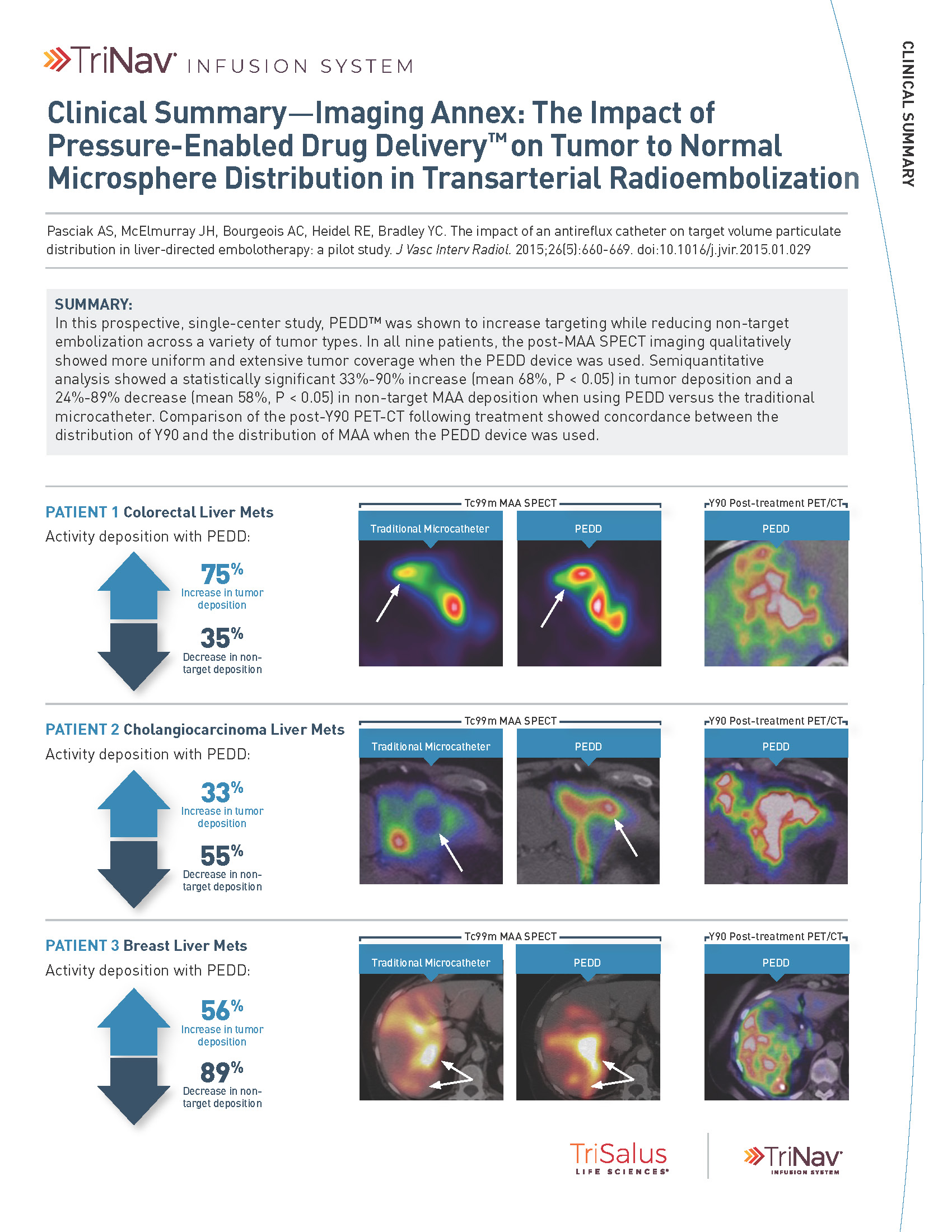
In a variety of tumor types, PEDD significantly increased tumor deposition and significantly decreased non-target embolization compared to a traditional microcatheter (TMC).
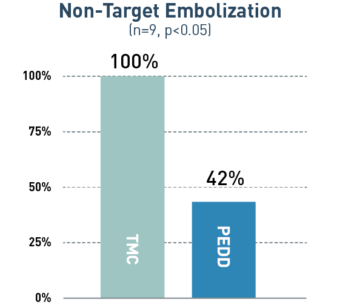
58%
Mean decrease in non-target embolization via PEDD
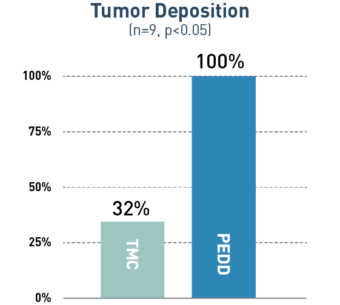
68%
Mean increase in tumor targeting via PEDD
Study Design
A prospective study included 9 patients who were referred for Y90 radioembolization treatment of their liver tumors. Prior to treatment delivered via PEDD, each patient received two same-day sequential lobar infusions of macroaggregated albumin (MAA) via traditional microcatheter and PEDD. Every infusion was performed from the same location, and post-MAA SPECT imaging was obtained. Differences in MAA distribution within the tumors and non-target sites were evaluated.
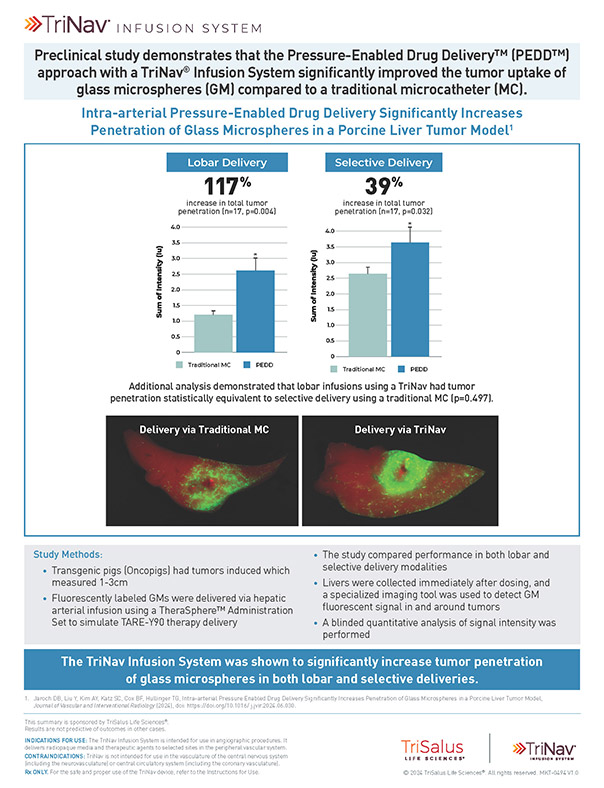
The TriNav Infusion System Significantly Increased Tumor Penetration of Glass Microspheres in Both Lobar and Selective Deliveries.5
In a porcine liver tumor model, TriNav with PEDD was shown to significantly improve glass microspheres (GM) uptake in liver tumors compared to a traditional microcatheter (TMC) in both lobar and selective infusions.
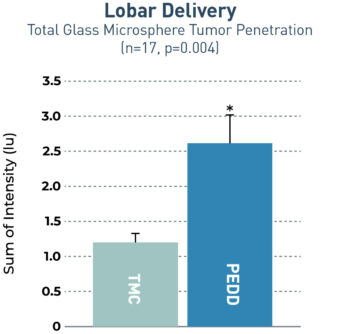
2.2x
increase in total tumor penetration via PEDD
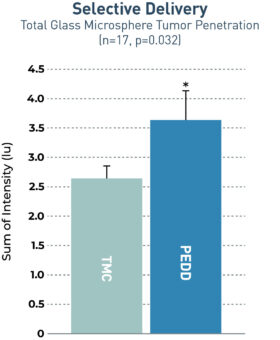
1.4x
increase in total tumor penetration via PEDD
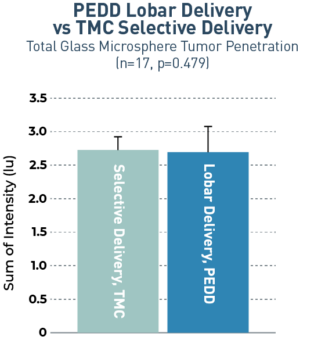
Lobar infusions via TriNav had statistically equivalent tumor penetration to selective delivery via TMC
Study Design
Transgenic pigs (Oncopigs) had tumors induced which measured 1-3 cm. Fluorescently labeled GMs were delivered via hepatic arterial infusion using a TheraSphere™ Administration Set to simulate TARE-Y90 therapy delivery. The study compared delivery via a TriNav to a TMC in both lobar and selective delivery positions. Livers were collected immediately after dosing, and a specialized imaging tool was used to detect GM fluorescent signal in and around tumors. A blinded quantitative analysis of signal intensity was performed.
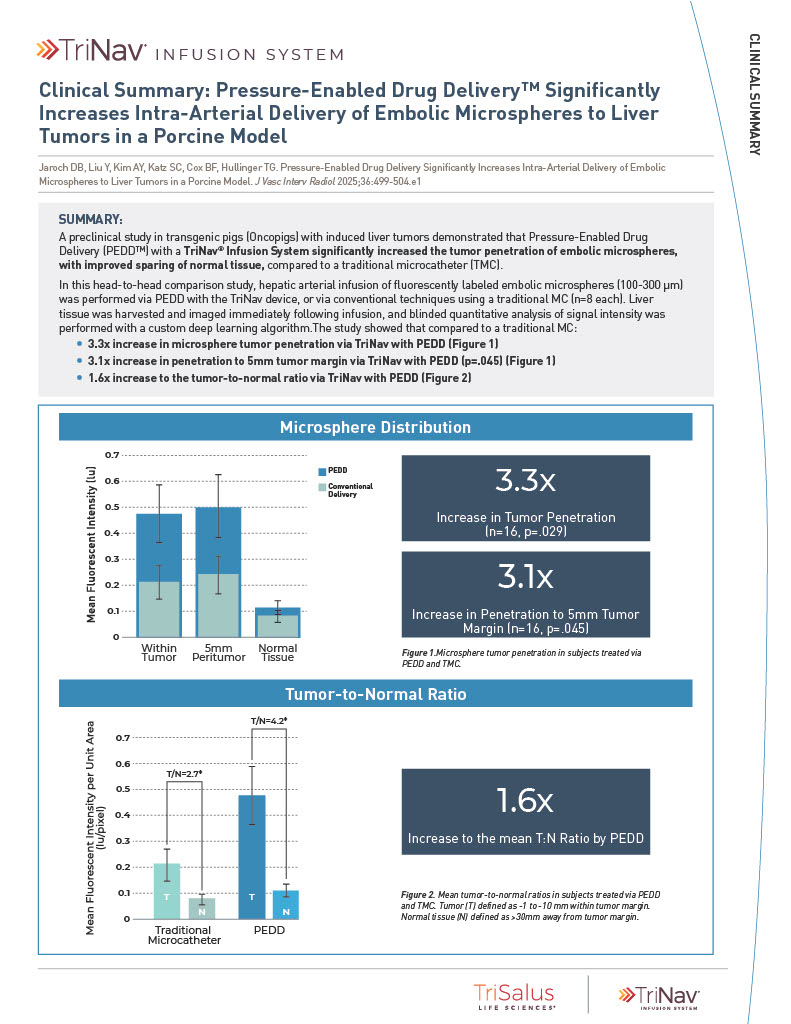
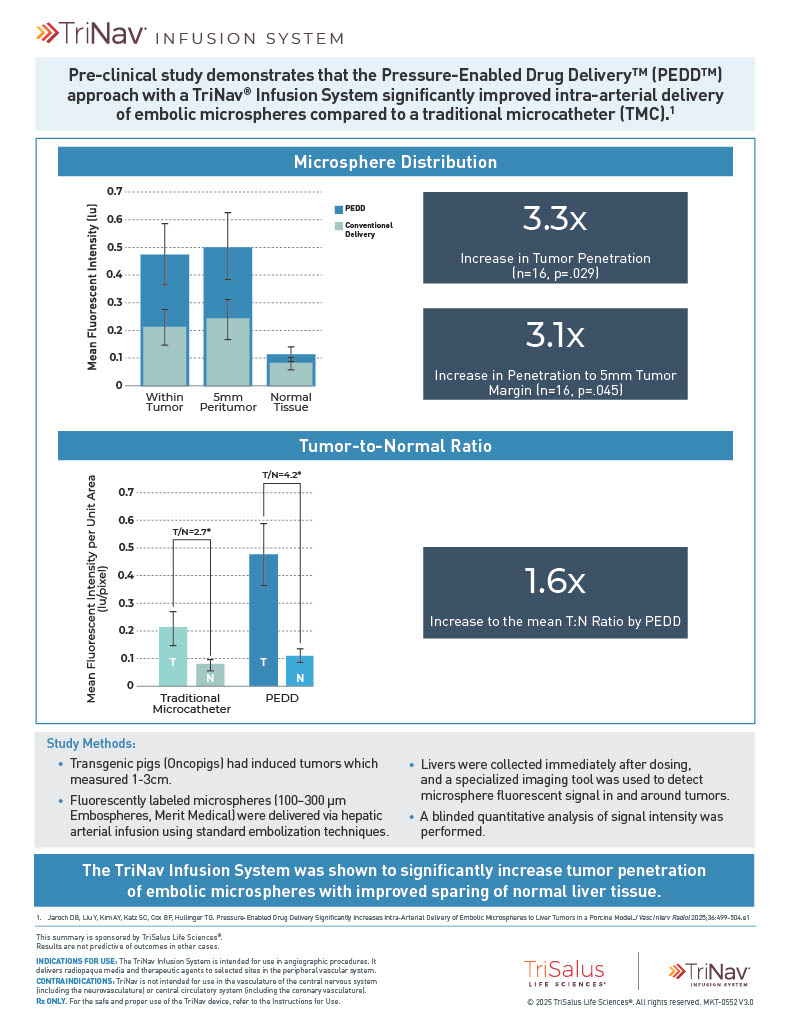
The TriNav Infusion System Significantly Increased Intra-Arterial Delivery of Embolic Microspheres to Liver Tumors in a Porcine Model⁷
In a porcine liver tumor model, TriNav with PEDD was shown to significantly increase the tumor penetration of embolic microspheres, with improved sparing of normal tissue, compared to a traditional microcatheter (TMC).
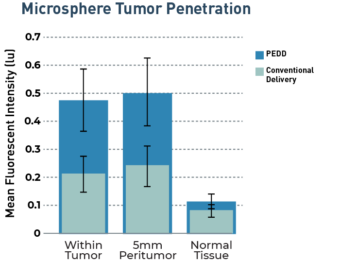
3.3x
increase in tumor penetration by PEDD (n=16, p=0.029)
3.1x
increase in penetration to 5mm tumor margin by PEDD (n=16, p=0.045)
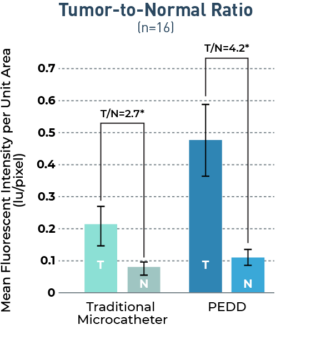
1.6x
increase to the mean T:N ratio by PEDD
Study Design
Transgenic pigs (Oncopigs) had tumors induced which measured 1-3 cm. Hepatic arterial infusion of fluorescently labeled embolic microspheres (100-300 μm) was performed via PEDD with the TriNav device, or via conventional techniques using a traditional MC (n=8 each). Liver tissue was harvested and imaged immediately following infusion, and blinded quantitative analysis of signal intensity was performed with a deep learning algorithm.
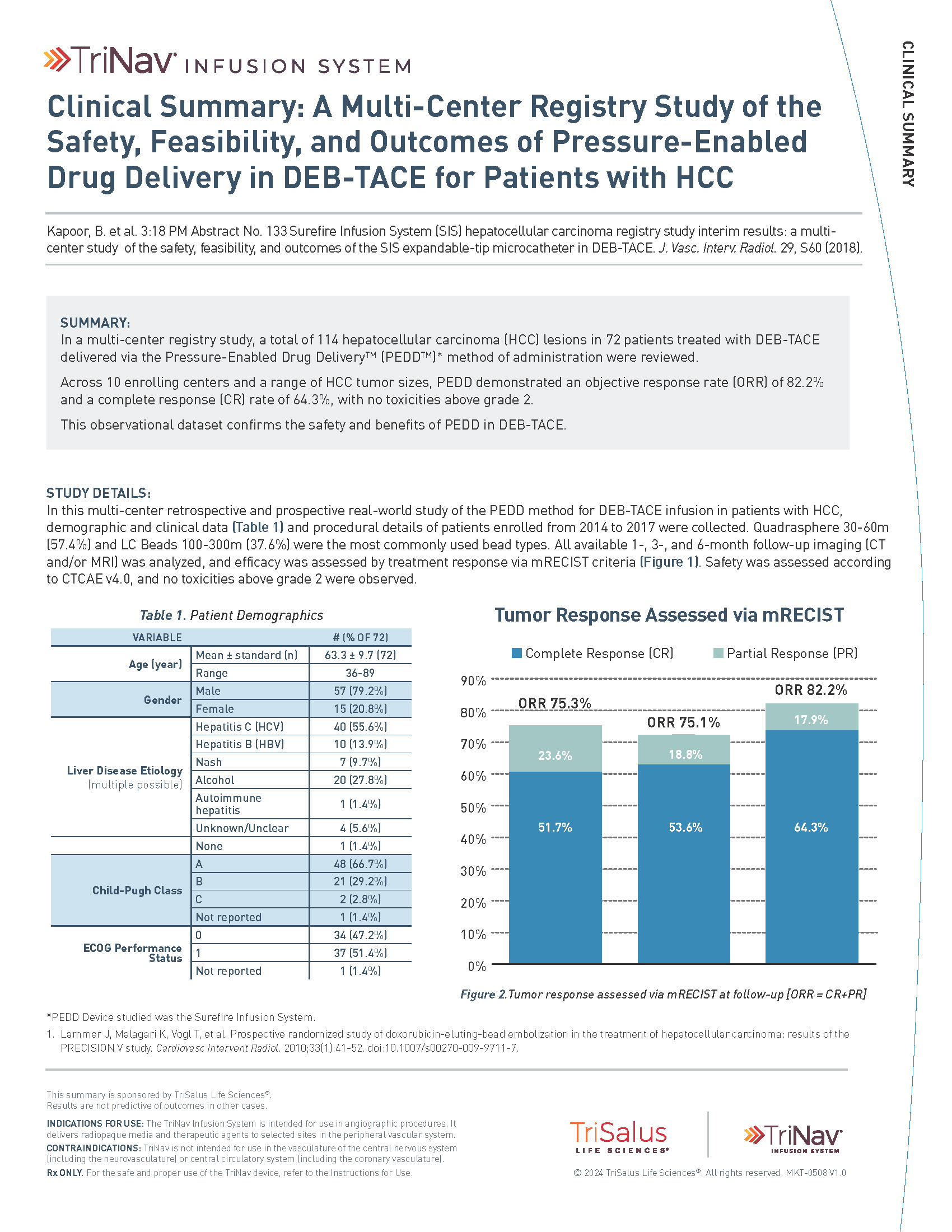
A Multi-Center Registry Study of the Safety, Feasibility, and Outcomes of PEDD in DEB-TACE for Patients with HCC.6
Across 10 enrolling centers and a range of HCC tumor sizes, PEDD demonstrated a 6-month objective response rate (ORR) of 82.2% and a complete response rate (CRR) of 64.3%, with no toxicities above grade.
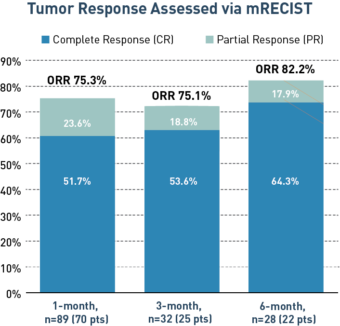
Study Design
A multi-center registry study in which 114 HCC lesions in 72 patients were treated with DEB-TACE delivered via the Pressure-Enabled Drug Delivery method from 2014 to 2017. All available 1, 3 and 6-month follow-up imaging (CT and/or MRI) was analyzed and efficacy was assessed by treatment response via mRECIST criteria. Safety was assessed according to CTCAE v4.0.
* Tumor to normal
References
1. Titano JJ, Fischman AM, Cherian A, et al. End-hole versus microvalve infusion catheters in patients undergoing drug-eluting microspheres–TACE for solitary hepatocellular carcinoma tumors: a retrospective analysis. Cardiovasc Intervent Radiol. 2019;42(4):560-568.
2. d’Abadie P, Walrand S, Goffette P, et al. Antireflux catheter improves tumor targeting in liver radioembolization with resin microspheres. Diagn Interv Radiol. 2021;27(6):768-773.
3. Pasciak AS, McElmurray JH, Bourgeois AC, Heidel RE, Bradley YC. The impact of an antireflux catheter on target volume particulate distribution in liver-directed embolotherapy: a pilot study. J Vasc Interv Radiol. 2015;26(5):660-669.
4. Cook K, Gupta D, Liu Y, et al. Real-world evidence of pressure-enabled drug delivery for trans-arterial chemoembolization and radioembolization among patients with hepatocellular carcinoma and liver metastases. Curr Med Res Opin. 2024;40(4):591-598.
5. Jaroch DB, Liu Y, Kim AY, Katz SC, Cox BF, Hullinger TG, Intra-arterial Pressure Enabled Drug Delivery Significantly Increases Penetration of Glass Microspheres in a Porcine Liver Tumor Model, Journal of Vascular and Interventional Radiology (2024), doi: https://doi.org/10.1016/ j.jvir.2024.06.030.
6. Kapoor, B. et al. 3:18 PM Abstract No. 133 Surefire Infusion System (SIS) hepatocellular carcinoma registry study interim results: a multicenter study of the safety, feasibility, and outcomes of the SIS expandable-tip microcatheter in DEB-TACE. J. Vasc. Interv. Radiol. 29, S60 (2018).
7. Jaroch DB, Liu Y, Kim AY, Katz SC, Cox BF, Hullinger TG. Pressure-Enabled Drug Delivery Significantly Increases Intra-Arterial Delivery of Embolic Microspheres to Liver Tumors in a Porcine Model. J Vasc Interv Radiol 2025;36:499-504.e1